A Public Health Antibody Screening Indicates a 6-Fold Higher SARS-CoV-2 Exposure Rate than Reported Cases in Children
- PMID: 33163984
- PMCID: PMC7598360
- DOI: 10.1016/j.medj.2020.10.003
A Public Health Antibody Screening Indicates a 6-Fold Higher SARS-CoV-2 Exposure Rate than Reported Cases in Children
Abstract
Background: Antibody responses to virus reflect exposure and potential protection.
Methods: We developed a highly specific and sensitive approach to measuring antibodies against SARS-CoV-2 for population-scale immune surveillance. Antibody positivity was defined as a dual-positive response against both the receptor-binding domain and nucleocapsid proteins of SARS-CoV-2. Antibodies were measured by immunoprecipitation assays in capillary blood from 15,771 children aged 1 to 18 years living in Bavaria, Germany, and participating in a public health type 1 diabetes screening program (ClinicalTrials.gov: NCT04039945), in 1,916 dried blood spots from neonates in a Bavarian screening study (ClinicalTrials.gov: NCT03316261), and in 75 SARS-CoV-2-positive individuals. Virus positive incidence was obtained from the Bavarian health authority data.
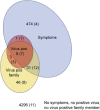
Findings: Dual-antibody positivity was detected in none of the 3,887 children in 2019 (100% specificity) and 73 of 75 SARS-CoV-2-positive individuals (97.3% sensitivity). Antibody surveillance in children during 2020 resulted in frequencies of 0.08% in January to March, 0.61% in April, 0.74% in May, 1.13% in June, and 0.91% in July. Antibody prevalence from April 2020 was 6-fold higher than the incidence of authority-reported cases (156 per 100,000 children), showed marked variation between the seven Bavarian regions (p < 0.0001), and was not associated with age or sex. Transmission in children with virus-positive family members was 35%. 47% of positive children were asymptomatic. No association with type 1 diabetes autoimmunity was observed. Antibody frequency in newborns was 0.47%.
Conclusions: We demonstrate the value of population-based screening programs for pandemic monitoring.
Funding: The work was supported by funding from the BMBF (FKZ01KX1818).
Keywords: RBD; SARS-CoV-2 antibody; nucleocapsid antigen; public health screening; receptor binding domain; seroprevalence; type 1 diabetes.
© 2020 Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. - PubMed
-
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. - PubMed
-
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., Adriano A., Beese S., Dretzke J., Ferrante di Ruffano L., et al. Cochrane COVID-19 Diagnostic Test Accuracy Group Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6:CD013652. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

