Endothelial cell apicobasal polarity coordinates distinct responses to luminally versus abluminally delivered TNF-α in a microvascular mimetic
- PMID: 33164044
- PMCID: PMC7671990
- DOI: 10.1093/intbio/zyaa022
Endothelial cell apicobasal polarity coordinates distinct responses to luminally versus abluminally delivered TNF-α in a microvascular mimetic
Abstract
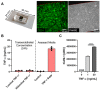
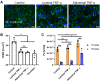
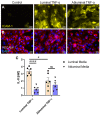
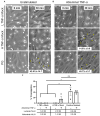
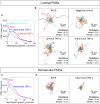
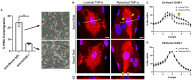
Endothelial cells (ECs) are an active component of the immune system and interact directly with inflammatory cytokines. While ECs are known to be polarized cells, the potential role of apicobasal polarity in response to inflammatory mediators has been scarcely studied. Acute inflammation is vital in maintaining healthy tissue in response to infection; however, chronic inflammation can lead to the production of systemic inflammatory cytokines and deregulated leukocyte trafficking, even in the absence of a local infection. Elevated levels of cytokines in circulation underlie the pathogenesis of sepsis, the leading cause of intensive care death. Because ECs constitute a key barrier between circulation (luminal interface) and tissue (abluminal interface), we hypothesize that ECs respond differentially to inflammatory challenge originating in the tissue versus circulation as in local and systemic inflammation, respectively. To begin this investigation, we stimulated ECs abluminally and luminally with the inflammatory cytokine tumor necrosis factor alpha (TNF-α) to mimic a key feature of local and systemic inflammation, respectively, in a microvascular mimetic (μSiM-MVM). Polarized IL-8 secretion and polymorphonuclear neutrophil (PMN) transmigration were quantified to characterize the EC response to luminal versus abluminal TNF-α. We observed that ECs uniformly secrete IL-8 in response to abluminal TNF-α and is followed by PMN transmigration. The response to abluminal treatment was coupled with the formation of ICAM-1-rich membrane ruffles on the apical surface of ECs. In contrast, luminally stimulated ECs secreted five times more IL-8 into the luminal compartment than the abluminal compartment and sequestered PMNs on the apical EC surface. Our results identify clear differences in the response of ECs to TNF-α originating from the abluminal versus luminal side of a monolayer for the first time and may provide novel insight into future inflammatory disease intervention strategies.
Keywords: apicobasal polarity; endothelial cells; inflammation; neutrophils; sepsis; tissue-chip.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permission@oup.com.
Figures

References
-
- Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med 1997;25:1095–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

