A Chemoenzymatic Synthon Strategy for Synthesizing N-Acetyl Analogues of O-Acetylated N. meningitidis W Capsular Polysaccharide Oligosaccharides
- PMID: 33164526
- PMCID: PMC7811823
- DOI: 10.1021/acs.joc.0c02134
A Chemoenzymatic Synthon Strategy for Synthesizing N-Acetyl Analogues of O-Acetylated N. meningitidis W Capsular Polysaccharide Oligosaccharides
Abstract
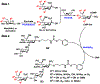
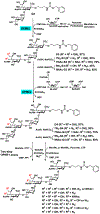
O-Acetylated sialic acid has been found in the Neisseria meningitidis serogroup W (NmW) capsular polysaccharide (CPS) and is a required structural component of clinically used NmW CPS-based polysaccharide and polysaccharide-conjugate vaccines. The role of sialic acid O-acetylation in NmW CPS, however, is not clearly understood. This is partially due to the lack of a precise control of the percentage and the location of O-acetylation which is labile and susceptible to migration. We explore chemoenzymatic synthetic strategies for preparing N-acetylated analogues of O-acetylated NmW CPS oligosaccharides which can serve as structurally stable probe mimics. Substrate specificity studies of NmW CPS polymerase (NmSiaDW) identified 4-azido-4-deoxy-N-acetylmannosamine (ManNAc4N3) and 6-azido-6-deoxy-N-acetylmannosamine (ManNAc6N3) as suitable chemoenzymatic synthons for synthesizing N-acetyl analogues of NmW CPS oligosaccharides containing 7-O-acetyl-N-acetylneuraminic acid (Neu5,7Ac2) and/or 9-O-acetyl-N-acetylneuraminic acid (Neu5,9Ac2). The synthesis was achieved by NmSiaDW-dependent sequential one-pot multienzyme (OPME) strategy with in situ generation of the corresponding sugar nucleotides from simple monosaccharides or derivatives to form N3-oligosaccharides which were converted to the desired NAc-oligosaccharides by an efficient one-step chemical transformation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
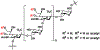
References
-
- Angata T; Varki A Chemical Diversity in The Sialic Acids and Related Alpha-Keto Acids: An Evolutionary Perspective. Chem. Rev 2002, 102, 439–469. - PubMed

