Patient Characteristics, Clinical Outcomes, and Effect of Dapagliflozin in Relation to Duration of Heart Failure: Is It Ever Too Late to Start a New Therapy?
- PMID: 33164553
- PMCID: PMC7610491
- DOI: 10.1161/CIRCHEARTFAILURE.120.007879
Patient Characteristics, Clinical Outcomes, and Effect of Dapagliflozin in Relation to Duration of Heart Failure: Is It Ever Too Late to Start a New Therapy?
Abstract
Background: The impact of heart failure (HF) duration on outcomes and treatment effect is largely unknown. We aim to compare baseline patient characteristics, outcomes, and the efficacy and safety of dapagliflozin, in relation to time from diagnosis of HF in DAPA-HF trial (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure).
Methods: HF duration was categorized as ≥2 to ≤12 months, >1 to 2 years, >2 to 5 years, and >5 years. Outcomes were adjusted for prognostic variables and analyzed using Cox regression. The primary end point was the composite of worsening HF or cardiovascular death. Treatment effect was examined within each duration category and by duration threshold.
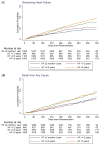
Results: The number of patients in each category was: 1098 (≥2-≤12 months), 686 (>1-2 years), 1105 (>2-5 years), and 1855 (>5 years). Longer-duration HF patients were older and more comorbid with worse symptoms. The rate of the primary outcome (per 100 person-years) increased with HF duration: 10.2 (95% CI, 8.7-12.0) for ≥2 to ≤12 months, 10.6 (8.7-12.9) >1 to 2 years, 15.5 (13.6-17.7) >2 to 5 years, and 15.9 (14.5-17.6) for >5 years. Similar trends were seen for all other outcomes. The benefit of dapagliflozin was consistent across HF duration and on threshold analysis. The hazard ratio for the primary outcome ≥2 to ≤12 months was 0.86 (0.63-1.18), >1 to 2 years 0.95 (0.64-1.42), >2 to 5 years 0.74 (0.57-0.96), and >5 years 0.64 (0.53-0.78), P interaction=0.26. The absolute benefit was greatest in longest-duration HF, with a number needed to treat of 18 for HF >5 years, compared with 28 for ≥2 to ≤12 months.
Conclusions: Longer-duration HF patients were older, had more comorbidity and symptoms, and higher rates of worsening HF and death. The benefits of dapagliflozin were consistent across HF duration. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03036124.
Keywords: comorbidity; dapagliflozin; heart failure; patients; prognosis.
Conflict of interest statement
Drs. Yeoh and Dewan have no conflicts of interest to disclose. Dr. Jhund’s employer (University of Glasgow) is paid by AstraZeneca for involvement in the DAPA-HF trial. He has also received consulting, advisory board, and speaker’s fees from Novartis, advisory board fees from Cytokinetics, and a grant from Boehringer Ingelheim; Dr. Inzucchi, received advisory fees from AstraZeneca and Zafgen, lecture fees, consulting fees, fees for serving as a clinical-trial publications committee member, reimbursement for medical writing, and travel support from Boehringer Ingelheim, fees for serving on a steering committee and travel support from Sanofi–Lexicon, lecture fees, consulting fees, and travel support from Merck, and advisory fees and travel support from vTv Therapeutics and Abbott–Alere; Dr. Køber, received lecture fees from Novartis and Bristol-Myers Squibb; Dr. Kosiborod, received grant support, honoraria, and research support from AstraZeneca, grant support and honoraria from Boehringer Ingelheim, and honoraria from Sanofi, Amgen, Novo Nordisk, Merck (Diabetes), Eisai, Janssen, Bayer, GlaxoSmithKline, Glytec, Intarcia Therapeutics, Novartis, Applied Therapeutics, Amarin, and Eli Lilly; Dr. Martinez has received personal fees from AstraZeneca as honoraria for being an Executive Committee Member for DAPA-HF. Dr. Ponikowski was an investigator in the DAPA-HF trial and received personal fees from AstraZeneca for lectures and consultancy related to the trial. He has also participated in clinical trials and received research grants to his institute and personal fees for speakers bureau and consultancy from Vifor Pharma. He has participated in clinical trials and received personal fees for consultancy and speakers bureaus from Boehringer Ingelheim, Bayer, Bristol Myers Squibb, Cibiem, Novartis, and RenalGuard, personal fees for speakers bureaus and consultancy from Servier and Respicardia, personal fees for speakers bureaus from Berlin-Chemie, and personal fees for lectures from Pfizer. Dr. Sabatine received grant support (paid to Brigham and Women’s Hospital) and consulting fees from Amgen, AstraZeneca, Intarcia Therapeutics, Janssen Research and Development, the Medicines Company, MedImmune, Merck, and Novartis, receiving consulting fees from Anthos Therapeutics, Bristol-Myers Squibb, CVS Caremark, DalCor Pharmaceuticals, Dyrnamix, Esperion, IFM Therapeutics, and Ionis Pharmaceuticals, receiving grant support (paid to Brigham and Woman’s Hospital) from Bayer, Daiichi Sankyo, Eisai, GlaxoSmithKline, Pfizer, Poxel, Quark Pharmaceuticals, and Takeda Pharmaceutical, and serving as a member of the TIMI Study Group, which receives grant support (paid to Brigham and Women’s Hospital) from Abbott, Aralez Pharmaceuticals, Roche, and Zora Biosciences; Dr Solomon has received a grant to his institution from AstraZeneca for being an Executive Committee Member for DAPA-HF and has received grants to his institute and personal fees for consulting from Alnylam, Amgen, AstraZeneca, Bristol Myers Squibb, Gilead, GlaxoSmithKline, MyoKardia, Novartis, Theracos, and Bayer, has received grants to his institute from Bellerophon, Celladon, Ionis, Lone Star Heart, Mesoblast, National Heart, Lung, and Blood Institute (National Institutes of Health), and Sanofi Pasteur, and personal fees for consulting from Akros, Corvia, Ironwood, Merck, Roche, Takeda, Quantum Genomics, and AoBiome. Drs. Bengtsson and Sjöstrand are employed by AstraZeneca; and Dr. Langkilde, is employed by and holds shares in AstraZeneca. Prof. McMurray’s employer has been paid by Cardiorentis for his time spent as Steering Committee member and Endpoint Committee Chair and related meetings, and he has received nonfinancial support for travel and accommodation for some related meetings. Prof. McMurray’s employer has been paid by Amgen, Oxford University/Bayer, Abbvie, and Bristol Myers Squibb for his time spent as a Steering Committee member and related meetings, and he has received nonfinancial support for travel and accommodation for some related meetings. Prof. McMurray’s employer has been paid by Kings College Hospital/Kidney Research UK/Vifor-Fresenius for his time spent as a Steering Committee member and for running an Endpoint Adjudication Committee and related meetings, and he has received nonfinancial support for travel and accommodation for some related meetings. Prof. McMurray’s employer has been paid by Theracos for his time spent as Principal Investigator and related meetings, and he has received nonfinancial support for travel and accommodation for some related meetings. Prof. McMurray’s employer has been paid by Pfizer and Merck for his time spent on the Data Safety Monitoring Committee and related meetings. Prof. McMurray’s employer has been paid by Novartis for his time spent as Executive/Steering Committee member, Co-Principal Investigator, and Advisory Board member, and he has received nonfinancial support for travel and accommodation for some related meetings/presentations. Prof. McMurray’s employer has been paid by Bayer for his participation as a Steering Committee member, by DalCor Pharmaceuticals for his participation as a Steering Committee member (and related meetings), and by Bristol Myers Squibb for his participation as a Steering Committee member (and related meetings). Prof. McMurray’s employer has been paid by GlaxoSmithKline for his participation as a Steering Committee member and Co-Principal Investigator, and he has received nonfinancial support for travel and accommodation for some related meetings. All payments for meetings-related travel and accommodation were made through a Consultancy with University of Glasgow, and Prof. McMurray has not received personal payments in relation to any trials/drugs.
Figures

Comment in
-
The Time Is Now for Sodium Glucose Co-Transporter 2 Inhibitors for Heart Failure: A Call to Overcome Clinical Inertia.Circ Heart Fail. 2020 Dec;13(12):e008030. doi: 10.1161/CIRCHEARTFAILURE.120.008030. Epub 2020 Nov 9. Circ Heart Fail. 2020. PMID: 33161728 No abstract available.
References
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. - PubMed
-
- Böhm M, Komajda M, Borer JS, Ford I, Maack C, Tavazzi L, Moyne A, Swedberg K. SHIFT Investigators. Duration of chronic heart failure affects outcomes with preserved effects of heart rate reduction with ivabradine: findings from SHIFT. Eur J Heart Fail. 2018;20:373–381. - PubMed
-
- Yeoh SE, Dewan P, Desai AS, Solomon SD, Rouleau JL, Lefkowitz M, Rizkala A, Swedberg K, Zile MR, Jhund PS, et al. Relationship between duration of heart failure, patient characteristics, outcomes and effect of therapy in PARADIGM-HF. ESC Heart Fail. 2020 doi: 10.1002/ehf2.12972. Epub ahead of print. - DOI - PMC - PubMed
-
- McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. DAPA-HF Committees and Investigators. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF) Eur J Heart Fail. 2019;21:665–675. - PMC - PubMed
-
- McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. DAPA-HF Committees and Investigators. The Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail. 2019;21:1402–1411. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

