Progression of infarct-mediated arrhythmogenesis in a rodent model of heart failure
- PMID: 33164577
- PMCID: PMC7847079
- DOI: 10.1152/ajpheart.00639.2020
Progression of infarct-mediated arrhythmogenesis in a rodent model of heart failure
Abstract
Heart failure (HF) post-myocardial infarction (MI) presents with increased vulnerability to monomorphic ventricular tachycardia (mmVT). To appropriately evaluate new therapies for infarct-mediated reentrant arrhythmia in the preclinical setting, chronologic characterization of the preclinical animal model pathophysiology is critical. This study aimed to evaluate the rigor and reproducibility of mmVT incidence in a rodent model of HF. We hypothesize a progressive increase in the incidence of mmVT as the duration of HF increases. Adult male Sprague-Dawley rats underwent permanent left coronary artery ligation or SHAM surgery and were maintained for either 6 or 10 wk. At end point, SHAM and HF rats underwent echocardiographic and invasive hemodynamic evaluation. Finally, rats underwent electrophysiologic (EP) assessment to assess susceptibility to mmVT and define ventricular effective refractory period (ERP). In 6-wk HF rats (n = 20), left ventricular (LV) ejection fraction (EF) decreased (P < 0.05) and LV end-diastolic pressure (EDP) increased (P < 0.05) compared with SHAM (n = 10). Ten-week HF (n = 12) revealed maintenance of LVEF and LVEDP (P > 0.05), (P > 0.05). Electrophysiology studies revealed an increase in incidence of mmVT between SHAM and 6-wk HF (P = 0.0016) and ERP prolongation (P = 0.0186). The incidence of mmVT and ventricular ERP did not differ between 6- and 10-wk HF (P = 1.0000), (P = 0.9831). Findings from this rodent model of HF suggest that once the ischemia-mediated infarct stabilizes, proarrhythmic deterioration ceases. Within the 6- and 10-wk period post-MI, no echocardiographic, invasive hemodynamic, or electrophysiologic changes were observed, suggesting stable HF. This is the necessary context for the evaluation of experimental therapies in rodent HF.NEW & NOTEWORTHY Rodent model of ischemic cardiomyopathy exhibits a plateau of inducible monomorphic ventricular tachycardia incidence between 6 and 10 wk postinfarction.
Keywords: adverse remodeling; ischemia; monophasic action potential; rigor and reproducibility; ventricular tachycardia.
Figures


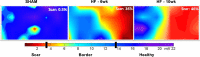

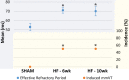
References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP , et al. Heart Disease and Stroke Statistics-2019 updated: a report from the American Heart Association. Circulation 139: e56–e528, 2019. [Erratum in Circulation 141: e33, 2020]. - PubMed
-
- Cutler MJ, Rosenbaum DS, Dunlap ME. Structural and electrical remodeling as therapeutic targets in heart failure. J Electrocardiol 40: S1–S7, 2007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

