Electrophysiological and Imaging Calcium Biomarkers of Aging in Male and Female 5×FAD Mice
- PMID: 33164928
- PMCID: PMC7836067
- DOI: 10.3233/JAD-200109
Electrophysiological and Imaging Calcium Biomarkers of Aging in Male and Female 5×FAD Mice
Abstract
Background: In animal models and tissue preparations, calcium dyshomeostasis is a biomarker of aging and Alzheimer's disease that is associated with synaptic dysfunction, neuritic pruning, and dysregulated cellular processes. It is unclear, however, whether the onset of calcium dysregulation precedes, is concurrent with, or is the product of pathological cellular events (e.g., oxidation, amyloid-β production, and neuroinflammation). Further, neuronal calcium dysregulation is not always present in animal models of amyloidogenesis, questioning its reliability as a disease biomarker.
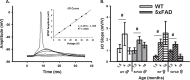
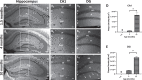
Objective: Here, we directly tested for the presence of calcium dysregulation in dorsal hippocampal neurons in male and female 5×FAD mice on a C57BL/6 genetic background using sharp electrodes coupled with Oregon-green Bapta-1 imaging. We focused on three ages that coincide with the course of amyloid deposition: 1.5, 4, and 10 months old.
Methods: Outcome variables included measures of the afterhyperpolarization, short-term synaptic plasticity, and calcium kinetics during synaptic activation. Quantitative analyses of spatial learning and memory were also conducted using the Morris water maze. Main effects of sex, age, and genotype were identified on measures of electrophysiology and calcium imaging.
Results: Measures of resting Oregon-green Bapta-1 fluorescence showed significant reductions in the 5×FAD group compared to controls. Deficits in spatial memory, along with increases in Aβ load, were detectable at older ages, allowing us to test for temporal associations with the onset of calcium dysregulation.
Conclusion: Our results provide evidence that reduced, rather than elevated, neuronal calcium is identified in this 5×FAD model and suggests that this surprising result may be a novel biomarker of AD.
Keywords: 5×FAD; Alzheimer’s disease; afterhyperpolarization; aging; calcium; electrophysiology; hippocampus; hyperactivity; intracellular; sex.
Conflict of interest statement
Authors’ disclosures available online (
Figures





References
-
- Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12, 459–509. - PubMed
-
- Mandelkow EM, Mandelkow E (1993) Tau as a marker for Alzheimer’s disease. Trends Biochem Sci 18, 480–483. - PubMed
-
- Lassmann H, Fischer P, Jellinger K (1993) Synaptic pathology of Alzheimer’s disease. Ann N Y Acad Sci 695, 59–64. - PubMed
-
- De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV (2012) Alzheimer’s disease. Subcell Biochem 65, 329–352. - PubMed
-
- Takeda S (2019) Progression of Alzheimer’s disease, tau propagation, and its modifiable risk factors. Neurosci Res 141, 36–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

