Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity
- PMID: 33164985
- PMCID: PMC7685757
- DOI: 10.1172/JCI141828
Decreased adipose tissue oxygenation associates with insulin resistance in individuals with obesity
Abstract
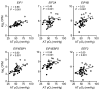
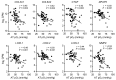
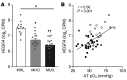
BACKGROUNDData from studies conducted in rodent models have shown that decreased adipose tissue (AT) oxygenation is involved in the pathogenesis of obesity-induced insulin resistance. Here, we evaluated the potential influence of AT oxygenation on AT biology and insulin sensitivity in people.METHODSWe evaluated subcutaneous AT oxygen partial pressure (pO2); liver and whole-body insulin sensitivity; AT expression of genes and pathways involved in inflammation, fibrosis, and branched-chain amino acid (BCAA) catabolism; systemic markers of inflammation; and plasma BCAA concentrations, in 3 groups of participants that were rigorously stratified by adiposity and insulin sensitivity: metabolically healthy lean (MHL; n = 11), metabolically healthy obese (MHO; n = 15), and metabolically unhealthy obese (MUO; n = 20).RESULTSAT pO2 progressively declined from the MHL to the MHO to the MUO group, and was positively associated with hepatic and whole-body insulin sensitivity. AT pO2 was positively associated with the expression of genes involved in BCAA catabolism, in conjunction with an inverse relationship between AT pO2 and plasma BCAA concentrations. AT pO2 was negatively associated with AT gene expression of markers of inflammation and fibrosis. Plasma PAI-1 increased from the MHL to the MHO to the MUO group and was negatively correlated with AT pO2, whereas the plasma concentrations of other cytokines and chemokines were not different among the MHL and MUO groups.CONCLUSIONThese results support the notion that reduced AT oxygenation in individuals with obesity contributes to insulin resistance by increasing plasma PAI-1 concentrations and decreasing AT BCAA catabolism and thereby increasing plasma BCAA concentrations.TRIAL REGISTRATIONClinicalTrials.gov NCT02706262.FUNDINGThis study was supported by NIH grants K01DK109119, T32HL130357, K01DK116917, R01ES027595, P42ES010337, DK56341 (Nutrition Obesity Research Center), DK20579 (Diabetes Research Center), DK052574 (Digestive Disease Research Center), and UL1TR002345 (Clinical and Translational Science Award); NIH Shared Instrumentation Grants S10RR0227552, S10OD020025, and S10OD026929; and the Foundation for Barnes-Jewish Hospital.
Keywords: Adipose tissue; Glucose metabolism; Metabolism; Obesity.
Conflict of interest statement
Figures





Comment in
-
Oxygenation of adipose tissue in insulin resistance.Nat Rev Endocrinol. 2021 Feb;17(2):68. doi: 10.1038/s41574-020-00457-y. Nat Rev Endocrinol. 2021. PMID: 33303950 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- T32 HL130357/HL/NHLBI NIH HHS/United States
- R01 ES027595/ES/NIEHS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- S10 OD020025/OD/NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- K01 DK116917/DK/NIDDK NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- S10 OD026929/OD/NIH HHS/United States
- K01 DK109119/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

