Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease
- PMID: 33165506
- PMCID: PMC7653537
- DOI: 10.1001/jamaneurol.2020.4201
Associations of Plasma Phospho-Tau217 Levels With Tau Positron Emission Tomography in Early Alzheimer Disease
Abstract
Importance: There is an urgent need for inexpensive and minimally invasive blood biomarkers for Alzheimer disease (AD) that could be used to detect early disease changes.
Objective: To assess how early in the course of AD plasma levels of tau phosphorylated at threonine 217 (P-tau217) start to change compared with levels of established cerebrospinal fluid (CSF) and positron emission tomography (PET) biomarkers of AD pathology.
Design, setting, and participants: This cohort study included cognitively healthy control individuals (n = 225) and participants with subjective cognitive decline (n = 89) or mild cognitive impairment (n = 176) from the BioFINDER-2 study. Participants were enrolled at 2 different hospitals in Sweden from January 2017 to October 2019. All study participants underwent plasma P-tau217 assessments and tau- and amyloid-β (Aβ)-PET imaging. A subcohort of 111 participants had 2 or 3 tau-PET scans.
Main outcomes and measures: Changes in plasma P-tau217 levels in preclinical and prodromal AD compared with changes in CSF P-tau217 and PET measures.
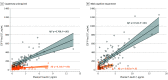
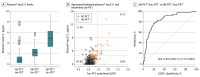
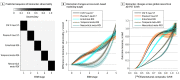
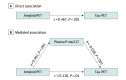
Results: Of 490 participants, 251 were women (51.2%) and the mean (SD) age was 65.9 (13.1) years. Plasma P-tau217 levels were increased in cognitively unimpaired participants with abnormal Aβ-PET but normal tau-PET in the entorhinal cortex (Aβ-PET+/ tau-PET- group vs Aβ-PET-/ tau-PET- group: median, 2.2 pg/mL [interquartile range (IQR), 1.5-2.9 pg/mL] vs 0.7 pg/mL [IQR, 0.3-1.4 pg/mL]). Most cognitively unimpaired participants who were discordant for plasma P-tau217 and tau-PET were positive for plasma P-tau217 and negative for tau-PET (P-tau217+/tau-PET-: 36 [94.7%]; P-tau217-/tau-PET+: 2 [5.3%]). Event-based modeling of cross-sectional data predicted that in cognitively unimpaired participants and in those with mild cognitive impairment, both plasma and CSF P-tau217 would change before the tau-PET signal in the entorhinal cortex, followed by more widespread cortical tau-PET changes. When testing the association with global Aβ load in nonlinear spline models, both plasma and CSF P-tau217 were increased at lower Aβ-PET values compared with tau-PET measures. Among participants with normal baseline tau-PET, the rates of longitudinal increase in tau-PET in the entorhinal cortex were higher in those with abnormal plasma P-tau217 at baseline (median standardized uptake value ratio, 0.029 [IQR, -0.006 to 0.041] vs -0.001 [IQR, -0.021 to 0.020]; Mann-Whitney U, P = .02).
Conclusions and relevance: In this cohort study, plasma P-tau217 levels were increased during the early preclinical stages of AD when insoluble tau aggregates were not yet detectable by tau-PET. Plasma P-tau217 may hold promise as a biomarker for early AD brain pathology.
Conflict of interest statement
Figures
Comment in
-
Rapid Progress Toward Reliable Blood Tests for Alzheimer Disease.JAMA Neurol. 2021 Feb 1;78(2):143-145. doi: 10.1001/jamaneurol.2020.4200. JAMA Neurol. 2021. PMID: 33165524 No abstract available.
References
-
- Tatebe H, Kasai T, Ohmichi T, et al. . Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and Down syndrome. Mol Neurodegener. 2017;12(1):63. doi:10.1186/s13024-017-0206-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

