Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial
- PMID: 33165621
- PMCID: PMC7653542
- DOI: 10.1001/jama.2020.22240
Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial
Abstract
Importance: Data on the efficacy of hydroxychloroquine for the treatment of coronavirus disease 2019 (COVID-19) are needed.
Objective: To determine whether hydroxychloroquine is an efficacious treatment for adults hospitalized with COVID-19.
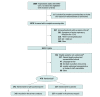
Design, setting, and participants: This was a multicenter, blinded, placebo-controlled randomized trial conducted at 34 hospitals in the US. Adults hospitalized with respiratory symptoms from severe acute respiratory syndrome coronavirus 2 infection were enrolled between April 2 and June 19, 2020, with the last outcome assessment on July 17, 2020. The planned sample size was 510 patients, with interim analyses planned after every 102 patients were enrolled. The trial was stopped at the fourth interim analysis for futility with a sample size of 479 patients.
Interventions: Patients were randomly assigned to hydroxychloroquine (400 mg twice daily for 2 doses, then 200 mg twice daily for 8 doses) (n = 242) or placebo (n = 237).
Main outcomes and measures: The primary outcome was clinical status 14 days after randomization as assessed with a 7-category ordinal scale ranging from 1 (death) to 7 (discharged from the hospital and able to perform normal activities). The primary outcome was analyzed with a multivariable proportional odds model, with an adjusted odds ratio (aOR) greater than 1.0 indicating more favorable outcomes with hydroxychloroquine than placebo. The trial included 12 secondary outcomes, including 28-day mortality.
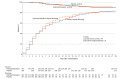
Results: Among 479 patients who were randomized (median age, 57 years; 44.3% female; 37.2% Hispanic/Latinx; 23.4% Black; 20.1% in the intensive care unit; 46.8% receiving supplemental oxygen without positive pressure; 11.5% receiving noninvasive ventilation or nasal high-flow oxygen; and 6.7% receiving invasive mechanical ventilation or extracorporeal membrane oxygenation), 433 (90.4%) completed the primary outcome assessment at 14 days and the remainder had clinical status imputed. The median duration of symptoms prior to randomization was 5 days (interquartile range [IQR], 3 to 7 days). Clinical status on the ordinal outcome scale at 14 days did not significantly differ between the hydroxychloroquine and placebo groups (median [IQR] score, 6 [4-7] vs 6 [4-7]; aOR, 1.02 [95% CI, 0.73 to 1.42]). None of the 12 secondary outcomes were significantly different between groups. At 28 days after randomization, 25 of 241 patients (10.4%) in the hydroxychloroquine group and 25 of 236 (10.6%) in the placebo group had died (absolute difference, -0.2% [95% CI, -5.7% to 5.3%]; aOR, 1.07 [95% CI, 0.54 to 2.09]).
Conclusions and relevance: Among adults hospitalized with respiratory illness from COVID-19, treatment with hydroxychloroquine, compared with placebo, did not significantly improve clinical status at day 14. These findings do not support the use of hydroxychloroquine for treatment of COVID-19 among hospitalized adults.
Trial registration: ClinicalTrials.gov: NCT04332991.
Conflict of interest statement
Figures

Comment in
-
Misguided Use of Hydroxychloroquine for COVID-19: The Infusion of Politics Into Science.JAMA. 2020 Dec 1;324(21):2161-2162. doi: 10.1001/jama.2020.22389. JAMA. 2020. PMID: 33165507 No abstract available.
References
-
- Johns Hopkins University Coronavirus resource center. Accessed July 28, 2020. https://coronavirus.jhu.edu/map.html
-
- World Health Organization WHO coronavirus disease (COVID-19) dashboard. Accessed September 22, 2020. https://covid19.who.int/
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR002538/TR/NCATS NIH HHS/United States
- U01 HL123018/HL/NHLBI NIH HHS/United States
- U01 HL123023/HL/NHLBI NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- U01 HL122989/HL/NHLBI NIH HHS/United States
- U01 HL123027/HL/NHLBI NIH HHS/United States
- T32 HL087738/HL/NHLBI NIH HHS/United States
- U01 HL123033/HL/NHLBI NIH HHS/United States
- U01 HL123022/HL/NHLBI NIH HHS/United States
- U01 HL123009/HL/NHLBI NIH HHS/United States
- U01 HL123031/HL/NHLBI NIH HHS/United States
- U01 HL123020/HL/NHLBI NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- U01 HL123008/HL/NHLBI NIH HHS/United States
- U01 HL123010/HL/NHLBI NIH HHS/United States
- K23 HL153584/HL/NHLBI NIH HHS/United States
- U01 HL123004/HL/NHLBI NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

