Maintenance agonist treatments for opiate-dependent pregnant women
- PMID: 33165953
- PMCID: PMC8094273
- DOI: 10.1002/14651858.CD006318.pub4
Maintenance agonist treatments for opiate-dependent pregnant women
Abstract
Background: The prevalence of opiate use among pregnant women can range from 1% to 2% to as high as 21%. Just in the United States alone, among pregnant women with hospital delivery, a fourfold increase in opioid use is reported from 1999 to 2014 (Haight 2018). Heroin crosses the placenta, and pregnant, opiate-dependent women experience a six-fold increase in maternal obstetric complications such as low birth weight, toxaemia, third trimester bleeding, malpresentation, puerperal morbidity, fetal distress and meconium aspiration. Neonatal complications include narcotic withdrawal, postnatal growth deficiency, microcephaly, neuro-behavioural problems, increased neonatal mortality and a 74-fold increase in sudden infant death syndrome. This is an updated version of the original Cochrane Review first published in 2008 and last updated in 2013.
Objectives: To assess the effectiveness of any maintenance treatment alone or in combination with a psychosocial intervention compared to no intervention, other pharmacological intervention or psychosocial interventions alone for child health status, neonatal mortality, retaining pregnant women in treatment, and reducing the use of substances.
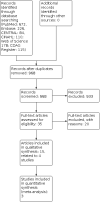
Search methods: We updated our searches of the following databases to February 2020: the Cochrane Drugs and Alcohol Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, and Web of Science. We also searched two trials registers and checked the reference lists of included studies for further references to relevant randomised controlled trials (RCTs).
Selection criteria: Randomised controlled trials which assessed the efficacy of any pharmacological maintenance treatment for opiate-dependent pregnant women.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane.
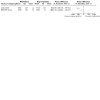
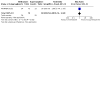
Main results: We found four trials with 271 pregnant women. Three compared methadone with buprenorphine and one methadone with oral slow-release morphine. Three out of four studies had adequate allocation concealment and were double-blind. The major flaw in the included studies was attrition bias: three out of four had a high dropout rate (30% to 40%), and this was unbalanced between groups. Methadone versus buprenorphine: There was probably no evidence of a difference in the dropout rate from treatment (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.37 to 1.20, three studies, 223 participants, moderate-quality evidence). There may be no evidence of a difference in the use of primary substances between methadone and buprenorphine (RR 1.81, 95% CI 0.70 to 4.68, two studies, 151 participants, low-quality evidence). Birth weight may be higher in the buprenorphine group in the two trials that reported data MD;-530.00 g, 95%CI -662.78 to -397.22 (one study, 19 particpants) and MD: -215.00 g, 95%CI -238.93 to -191.07 (one study, 131 participants) although the results could not be pooled due to very high heterogeneity (very low-quality of evidence). The third study reported that there was no evidence of a difference. We found there may be no evidence of a difference in the APGAR score (MD: 0.00, 95% CI -0.03 to 0.03, two studies,163 participants, low-quality evidence). Many measures were used in the studies to assess neonatal abstinence syndrome. The number of newborns treated for neonatal abstinence syndrome, which is the most critical outcome, may not differ between groups (RR 1.19, 95% CI 0.87 to1.63, three studies, 166 participants, low-quality evidence). Only one study which compared methadone with buprenorphine reported side effects. We found there may be no evidence of a difference in the number of mothers with serious adverse events (AEs) (RR 1.69, 95% CI 0.75 to 3.83, 175 participants, low-quality evidence) and we found there may be no difference in the numbers of newborns with serious AEs (RR 4.77, 95% CI 0.59, 38.49,131 participants, low-quality evidence). Methadone versus slow-release morphine: There were no dropouts in either treatment group. Oral slow-release morphine may be superior to methadone for abstinence from heroin use during pregnancy (RR 2.40, 95% CI 1.00 to 5.77, one study, 48 participants, low-quality evidence). In the comparison between methadone and slow-release morphine, no side effects were reported for the mother. In contrast, one child in the methadone group had central apnoea, and one child in the morphine group had obstructive apnoea (low-quality evidence).
Authors' conclusions: Methadone and buprenorphine may be similar in efficacy and safety for the treatment of opioid-dependent pregnant women and their babies. There is not enough evidence to make conclusions for the comparison between methadone and slow-release morphine. Overall, the body of evidence is too small to make firm conclusions about the equivalence of the treatments compared. There is still a need for randomised controlled trials of adequate sample size comparing different maintenance treatments.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
None.
Figures
















Update of
-
Maintenance agonist treatments for opiate-dependent pregnant women.Cochrane Database Syst Rev. 2013 Dec 23;(12):CD006318. doi: 10.1002/14651858.CD006318.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Nov 9;11:CD006318. doi: 10.1002/14651858.CD006318.pub4. PMID: 24366859 Updated.
References
References to studies included in this review
Fischer 1999 {published data only}
-
- Fischer G, Jagsch R, Eder H, Gombas W, Etzerdorfer P, Schmidl-Mohl K, et al. Comparison of methadone and slow-release morphine maintenance in pregnant addicts. Addiction 1999;94(2):231-9. - PubMed
Fischer 2006 {published data only}
-
- Fischer G, Ortner R, Rohrmeister K, Jagsch R, Baewert A, Langer M, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double dummy comparison study. Addiction 2006;101(2):275-81. - PubMed
Jones 2005 {published data only}
-
- Jones HE, Johnson RE, Jasinski DR, O'Grady KE, Chisholm CA, Choo RE, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug and Alcohol Dependence 2005;79(1):1-10. - PubMed
MOTHER Study {published data only}
References to studies excluded from this review
Bandstra 2012 {published data only}
-
- Bandstra, Emmalee S. Maternal Opioid Treatment: Human Experimental Research (MOTHER) study: maternal, fetal and neonatal outcomes from secondary analyses. Addiction 2012;107(Suppl 1):1-4. - PubMed
Bell 2007 {published data only}
-
- Bell J, Zador D. Dihydrocodeine as effective as methadone for maintenance of treatment for opiate dependence? Evidence Based Mental Health 2007;10(3):88. - PubMed
Binder 2008 {published data only}
-
- Binder T, Vavrinkova B. Prospective randomised comparative study of the effect of buprenorphine, methadone and heroin on the course of pregnancy, birthweight of newborns, early postpartum adaptation and course of the neonatal abstinence syndrome (NAS) in women followed up in the outpatient department. Neuro Endocrinology Letters 2008;29(1):80-6. - PubMed
Carroll 1995 {published data only}
-
- Carroll KM, Chang G, Behr H, Clinton B, Kosten TR. Improving treatment outcome in pregnant, methadone-maintained women. American Journal on Addictions 1995;4(1):56-9.
Cochran 2019 {published data only}
Dawe 2007 {published data only}
-
- Dawe S, Harnett P. Reducing potential for child abuse among methadone-maintained parents: results from a randomized controlled trial. Journal of Substance Abuse Treatment 2007;32(4):381-90. - PubMed
Ebner 2007 {published data only}
-
- Ebner N, Rohrmeister K, Winklbaur B, Baewert A, Jagsch R, Peternell A, et al. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug and Alcohol Dependence 2007;87:131-8. - PubMed
Fisher 1998 {published data only}
-
- Fisher G, Etzersdorfer P, Eder H, Jagsch R, Langer M, Weninger M. Buprenorphine maintenance in pregnant opiate addicts. European Addiction Research 1998;1:32-6. - PubMed
Gordon 2004 {published data only}
-
- Gordon AL, Stacey H, Pearson V, Haslam RR, Lopatko OV, White JM. Buprenorphine and methadone in pregnancy: effects on the mother and fetus/neonate. In: Sixty-Sixth Annual Scientific Meeting of the College on Problems of Drug Dependence. 2004.
Hulse 2004 {published data only}
-
- Hulse GK, O'Neil G, Arnold-Reed DE. Methadone maintenance vs. implantable naltrexone treatment in the pregnant heroin user. International Journal of Gynaecology & Obstetrics 2004;85(2):170-1. - PubMed
Jackson 2004 {published data only}
Jones 2008 {published data only}
Jones 2011 {published data only}
Keyser‐Marcus 2002 {published data only}
-
- Keyser-Marcus L, Miles D, Jansson L, Jones H, Svikis D. Perinatal opiate dependence: methadone and birth outcomes. In: Drug and Alcohol Dependence. Vol. 66 Suppl 1. 2002.
Lacroix 2011 {published data only}
-
- Lacroix I, Berrebi A, Garipuy D, Schmitt L, Hammou Y, Chaumerliac C, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. European Journal of Clinical Pharmacology 2011;67(10):1053-9. - PubMed
Laken 1997 {published data only}
-
- Laken MP, McComish JF, Ager J. Predictors of prenatal substance use and birth weight during outpatient treatment. Journal of Substance Abuse Treatment 1997;14(4):359-66. - PubMed
Martin 2011 {published data only}
-
- Martin PR. Opioid dependence during pregnancy: balancing risk versus benefit. Klinik Psikofarmakoloji Bülteni 2011;21:S35.
Newman 2009 {published data only}
-
- Newman R. Response to "Methadone maintenance vs. methadone taper during pregnancy" paper. American Journal on Addictions 2009;18(3):250; author reply 251. - PubMed
Stine 2009 {published data only}
Suchman 2007 {published data only}
Tuten 2012 {published data only}
-
- Tuten M, Svikis DS, Keyser-Marcus L, O'Grady KE, Jones HE. Lessons learned from a randomized trial of fixed and escalating contingency management schedules in opioid-dependent pregnant women. American Journal of Drug and Alcohol Abuse 2012;38(4):286-92. - PubMed
References to ongoing studies
NCT 03098407 {published data only}
-
- NCT03098407. Opioid dependence treatment therapies in pregnancy [A pilot randomized comparative effectiveness clinical trial of buprenorphine vs. methadone for the treatment of opioid dependence in pregnancy]. clinicaltrials.gov/show/NCT03098407 (first received 31 March 2017).
Additional references
AIHW 2011
-
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey detailed report 2010. Canberra: Australian Institute of Health and Welfare. www.aihw.gov.au/reports/illicit-use-of-drugs/2010-ndshs/contents/table-o... (accessed 23 March 2020).
AIHW 2014
-
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey detailed report 2013. www.aihw.gov.au/reports/illicit-use-of-drugs/2013-ndshs-detailed/content... (accessed 23 March 2020).
Amato 2011
-
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD004147. [DOI: 10.1002/14651858.CD004147.pub4] - DOI - PubMed
Behnke 2013
Clark 2002
CSAT 2005
-
- Center for Substance Abuse Treatment. Medication-assisted treatment for opioid addiction in opioid treatment programs. (Treatment Improvement Protocol Series 43). www.ncbi. nlm.nih.gov 2005. - PubMed
Dattel 1990
-
- Dattel B. Substance abuse in pregnancy. Seminars in Perinatology 1990;14(2):179-87. - PubMed
Dunlop 2003
-
- Dunlop AJ, Panjari M, O'Sullivan H, Henschke P, Love V et al. Clinical guidelines for the use of buprenorphine in pregnancy. Turning Point Alcohol and Drug Centre, Fitzroy, Australia 2003.
Egger 1997
EMCDDA 2014
-
- European Monitoring Centre for Drugs and Drug Addiction. Pregnancy and opioid use: strategies for treatment. In: EMCDDA Papers. Luxembourg: Publications Office of the European Union, 2014.
Faggiano 2003
Fajemirokun 2006
-
- Fajemirokun-Odudeyi O, Sinha C, Tutty S, Pairaudeau P, Armstrong D, Phillips T, et al. Pregnancy outcome in women who use opiates. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2006;126(2):170-5. - PubMed
Ferri 2011
Finnegan 1992
-
- Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Primary Pediatric Care. 2nd edition. St Louis: CV Mosby, 1992.
Haight 2018
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hulse 1997
-
- Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction 1997;92(11):1571-9. - PubMed
Hulse 1998
-
- Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal opiate use and neonatal mortality. Addiction 1998;93(7):1033-42. - PubMed
Jarvis 1994
-
- Jarvis MA, Schnoll SH. Methadone treatment during pregnancy. Journal of Psychoactive Drugs 1994;26(2):155-61. - PubMed
Johnson 2003
-
- Johnson RE, Jones HE, Fisher G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug and Alcohol Dependence 2003;70:S87-101. - PubMed
Jones 2012
Kaltenbach 1998
-
- Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. Obstetrics and Gynecology Clinics of North America 1998;25:139-51. - PubMed
Klaman 2017
-
- Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, et al. Treating women who are pregnant and parenting for opioid use disorder and the concurrent care of their infants and children: literature review to support national guidance. Journal of Addiction Medicine 2017;11:178-90. - PMC - PubMed
Lejuene 2006
-
- Lejuene C, Simmat-Durand L, Gourarier L, Aubisson S for the Groupe d'Etudes Grossesses et Addictions (GEGA). Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug and Alcohol Dependence 2006;82(3):250-7. - PubMed
Ludlow 2004
-
- Ludlow JP, Evans SF, Hulse G. Obstetric and perinatal outcomes in pregnancies associated with illicit substance abuse. Australian and New Zealand Journal of Obstetrics and Gynaecology 2004;44(4):301-6. - PubMed
Mattick 2008
Mattick 2009
Mayet 2004
Minozzi 2011
Monitoring the future 2017
-
- National Institute of Drug Abuse. Monitoring the future: treating opioid use disorder during pregnancy. www.drugabuse.gov/publications/treating-opioid-use-disorder-during-pregn... (accessed prior to 8 October 2020).
NIH 1998
-
- National Institutes of Health Consensus Development Panel. Effective medical treatment of opiate addiction. JAMA 1998;280(22):1936-43. - PubMed
ONDCP 2020
-
- Office of National Drug Control Policy. National Drug Control Strategy. Washington, D.C: Office of National Drug Control Policy, Executive Office of the President, 2020.
Ordean 2018
-
- Ordean A. Opioid agonist treatment during pregnancy: review of methadone and buprenorphine. In: Best Start Resource Centre Annual Conference; 2018 February 7. en.beststart.org/2018-bsrc-conference-handouts, 2018.
Rayburn 2004
-
- Rayburn W, Bogenschutz MP. Pharmacotherapy for pregnant women with addiction. American Journal of Obstetrics and Gynecology 2004;191:1885-97. - PubMed
Reddy 2017
-
- Reddy UM, Davis JM, Ren Z, Greene MF, Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes Workshop invited speakers. Opioid use in pregnancy, neonatal abstinence syndrome, and childhood outcomes: executive summary of a joint workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American Congress of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstetrics and Gynecology 2017;130(1):10-28. - PMC - PubMed
Schunemann 2013
-
- Schunemann H, Brozek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
UK Guidelines 2007
-
- Department of Health (England), The Scottish Government, Welsh Assembly Government and Northern Ireland Executive. Drug Misuse and Dependence: UK Guidelines on Clinical Management. www.smmgp.org.uk 2007.
UNODC 2018
-
- UNODC/WHO. World Drug Report 2018. www.unodc.org/wdr2018/ (accessed prior to 8 October 2020).
Wang 1999
-
- Wang EC. Methadone treatment during pregnancy. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1999;28:615-22. - PubMed
Wang 2018
-
- Wang M. Opioid detoxification during pregnancy: systematic review and meta-analysis of maternal and neonatal outcomes. Obstetrics & Gynecology 2018;131:197s.
WHO 2014
-
- World Health Organization. Guidelines for the Identification and Management of Substance Use and Substance Use Disorders in Pregnancy. Geneva, Switzerland: World Health Organization, 2014. - PubMed
Winklbaur 2008
-
- Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction 2008;103(9):1429-40. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

