Gqα/G11α deficiency in dorsomedial hypothalamus leads to obesity resulting from decreased energy expenditure and impaired sympathetic nerve activity
- PMID: 33166186
- PMCID: PMC8260363
- DOI: 10.1152/ajpendo.00059.2020
Gqα/G11α deficiency in dorsomedial hypothalamus leads to obesity resulting from decreased energy expenditure and impaired sympathetic nerve activity
Abstract
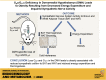
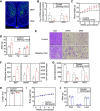
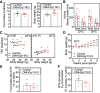
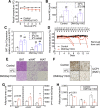
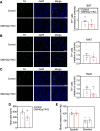
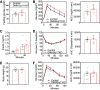
The G-protein subunits Gqα and G11α (Gq/11α) couple receptors to phospholipase C, leading to increased intracellular calcium. In this study we investigated the consequences of Gq/11α deficiency in the dorsomedial hypothalamus (DMH), a critical site for the control of energy homeostasis. Mice with DMH-specific deletion of Gq/11α (DMHGq/11KO) were generated by stereotaxic injection of adeno-associated virus (AAV)-Cre-green fluorescent protein (GFP) into the DMH of Gqαflox/flox:G11α-/- mice. Compared with control mice that received DMH injection of AAV-GFP, DMHGq/11KO mice developed obesity associated with reduced energy expenditure without significant changes in food intake or physical activity. DMHGq/11KO mice showed no defects in the ability of the melanocortin agonist melanotan II to acutely stimulate energy expenditure or to inhibit food intake. At room temperature (22°C), DMHGq/11KO mice showed reduced sympathetic nervous system activity in brown adipose tissue (BAT) and heart, accompanied with decreased basal BAT uncoupling protein 1 (Ucp1) gene expression and lower heart rates. These mice were cold intolerant when acutely exposed to cold (6°C for 5 h) and had decreased cold-stimulated BAT Ucp1 gene expression. DMHGq/11KO mice also failed to adapt to gradually declining ambient temperatures and to develop adipocyte browning in inguinal white adipose tissue although their BAT Ucp1 was proportionally stimulated. Consistent with impaired cold-induced thermogenesis, the onset of obesity in DMHGq/11KO mice was significantly delayed when housed under thermoneutral conditions (30°C). Thus our results show that Gqα and G11α in the DMH are required for the control of energy homeostasis by stimulating energy expenditure and thermoregulation.NEW & NOTEWORTHY This paper demonstrates that signaling within the dorsomedial hypothalamus via the G proteins Gqα and G11α, which couple cell surface receptors to the stimulation of phospholipase C, is critical for regulation of energy expenditure, thermoregulation by brown adipose tissue and the induction of white adipose tissue browning.
Keywords: G proteins; hypothalamus; obesity; thermogenesis.
Figures
Similar articles
-
Gsα deficiency in the dorsomedial hypothalamus leads to obesity, hyperphagia, and reduced thermogenesis associated with impaired leptin signaling.Mol Metab. 2019 Jul;25:142-153. doi: 10.1016/j.molmet.2019.04.005. Epub 2019 Apr 12. Mol Metab. 2019. PMID: 31014927 Free PMC article.
-
Susceptibility to diet-induced obesity at thermoneutral conditions is independent of UCP1.Am J Physiol Endocrinol Metab. 2022 Feb 1;322(2):E85-E100. doi: 10.1152/ajpendo.00278.2021. Epub 2021 Dec 20. Am J Physiol Endocrinol Metab. 2022. PMID: 34927460
-
Central FGF19 signaling enhances energy homeostasis and adipose tissue thermogenesis through sympathetic activation in obese mice.Am J Physiol Endocrinol Metab. 2025 Apr 1;328(4):E524-E542. doi: 10.1152/ajpendo.00488.2024. Epub 2025 Mar 10. Am J Physiol Endocrinol Metab. 2025. PMID: 40059865
-
Role of leptin in energy expenditure: the hypothalamic perspective.Am J Physiol Regul Integr Comp Physiol. 2017 Jun 1;312(6):R938-R947. doi: 10.1152/ajpregu.00045.2016. Epub 2017 Mar 29. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28356295 Review.
-
[Dapagliflozin, a Sodium-Glucose Co-transporter-2 Inhibitor, Acutely Reduces Energy Expenditure in Brown Adipose Tissue via Neural Signals in Mice].Yakugaku Zasshi. 2018;138(7):945-954. doi: 10.1248/yakushi.17-00223-3. Yakugaku Zasshi. 2018. PMID: 29962474 Review. Japanese.
Cited by
-
Hypothalamic neural circuits regulating energy expenditure.Vitam Horm. 2025;127:79-124. doi: 10.1016/bs.vh.2024.07.004. Epub 2024 Jul 20. Vitam Horm. 2025. PMID: 39864947 Free PMC article. Review.
-
A human obesity-associated MC4R mutation with defective Gq/11α signaling leads to hyperphagia in mice.J Clin Invest. 2024 Jan 4;134(4):e165418. doi: 10.1172/JCI165418. J Clin Invest. 2024. PMID: 38175730 Free PMC article.
-
RIIβ-PKA in GABAergic Neurons of Dorsal Median Hypothalamus Governs White Adipose Browning.Adv Sci (Weinh). 2023 Feb;10(5):e2205173. doi: 10.1002/advs.202205173. Epub 2022 Dec 18. Adv Sci (Weinh). 2023. PMID: 36529950 Free PMC article.
-
Hypothalamic GPCR Signaling Pathways in Cardiometabolic Control.Front Physiol. 2021 Jun 28;12:691226. doi: 10.3389/fphys.2021.691226. eCollection 2021. Front Physiol. 2021. PMID: 34262481 Free PMC article. Review.
References
-
- Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci 24: 5370–5380, 2004. doi:10.1523/JNEUROSCI.1219-04.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

