Neural mass modeling of slow-fast dynamics of seizure initiation and abortion
- PMID: 33166277
- PMCID: PMC7676664
- DOI: 10.1371/journal.pcbi.1008430
Neural mass modeling of slow-fast dynamics of seizure initiation and abortion
Abstract
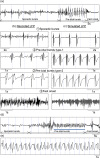
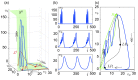
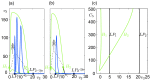
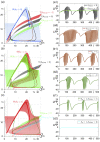
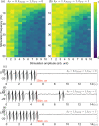
Epilepsy is a dynamic and complex neurological disease affecting about 1% of the worldwide population, among which 30% of the patients are drug-resistant. Epilepsy is characterized by recurrent episodes of paroxysmal neural discharges (the so-called seizures), which manifest themselves through a large-amplitude rhythmic activity observed in depth-EEG recordings, in particular in local field potentials (LFPs). The signature characterizing the transition to seizures involves complex oscillatory patterns, which could serve as a marker to prevent seizure initiation by triggering appropriate therapeutic neurostimulation methods. To investigate such protocols, neurophysiological lumped-parameter models at the mesoscopic scale, namely neural mass models, are powerful tools that not only mimic the LFP signals but also give insights on the neural mechanisms related to different stages of seizures. Here, we analyze the multiple time-scale dynamics of a neural mass model and explain the underlying structure of the complex oscillations observed before seizure initiation. We investigate population-specific effects of the stimulation and the dependence of stimulation parameters on synaptic timescales. In particular, we show that intermediate stimulation frequencies (>20 Hz) can abort seizures if the timescale difference is pronounced. Those results have the potential in the design of therapeutic brain stimulation protocols based on the neurophysiological properties of tissue.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Dudek FE, Staley KJ. The Time Course and Circuit Mechanisms of Acquired Epileptogenesis. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): National Center for Biotechnology Information (US); 2012. - PubMed

