Intranasal GSK2245035, a Toll-like receptor 7 agonist, does not attenuate the allergen-induced asthmatic response in a randomized, double-blind, placebo-controlled experimental medicine study
- PMID: 33166307
- PMCID: PMC7652256
- DOI: 10.1371/journal.pone.0240964
Intranasal GSK2245035, a Toll-like receptor 7 agonist, does not attenuate the allergen-induced asthmatic response in a randomized, double-blind, placebo-controlled experimental medicine study
Abstract
Background: Allergic asthma is a heterogenous disorder predominantly driven by a type 2 inflammatory response to aeroallergens. Therapeutic modulation to rebalance these type 2 responses may offer clinical benefit for allergic respiratory inflammatory diseases, with the potential for disease modification. GSK2245035, a selective toll-like receptor-7 agonist, preferentially stimulates the induction of type 1 interferon alpha, reducing type 2 responses.
Objective: This study investigated whether intranasal GSK2245035 reduced allergen-induced bronchial reactivity in mild allergic asthma.
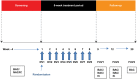
Methods: This double-blind, placebo-controlled, parallel-group Phase IIa trial randomized (1:1) participants with mild allergic asthma to intranasal GSK2245035 20 ng or placebo once weekly for 8 weeks; follow-up was conducted 1, 4, and 12 weeks after treatment. Allergen-induced late asthmatic response 1 week after treatment was measured as minimum and weighted mean forced expiratory volume in 1 second (FEV1) 4-10 hours following bronchial allergen challenge (primary endpoint). Pharmacodynamic and allergic biomarkers, and adverse events, were assessed. A Bayesian analysis framework was used; a posterior probability >0.7 denoted primary endpoint success.
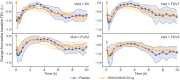
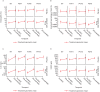
Results: Thirty-six participants were randomized (GSK2245035, n = 22; placebo, n = 14). The percentage attenuation in late asthmatic response was -4.6% (posterior probability: 0.385) and -10.5% (posterior probability: 0.303) for minimum and weighted mean FEV1, respectively. Type 2 responses were confirmed by changes in lung function, eosinophils (blood and sputum), interleukin-5 (sputum) and fractional exhaled nitric oxide biomarkers pre- and post-bronchial allergen challenge. However, no treatment effect was observed. Adverse events were reported by 10/14 (71%) and 21/22 (95%) participants in the placebo and GSK2245035 groups, respectively; headache was the most common.
Conclusions and clinical relevance: Although target engagement was observed, weekly intranasal GSK2245035 20 ng for 8 weeks did not substantially attenuate the late asthmatic response in participants with mild allergic asthma. Overall, treatment was well tolerated.
Trial registration: ClinicalTrials.gov NCT02833974.
Conflict of interest statement
HS, WP, and SS are employees of GSK and hold shareholder status in the company. DQ, HP and LL were employees of GSK and held shareholder status at the time of study conduct. JMH’s institution received funding from GSK for study conduct. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Therevance and Verona. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures