A 3-dimensional microfluidic platform for modeling human extravillous trophoblast invasion and toxicological screening
- PMID: 33166377
- PMCID: PMC8212566
- DOI: 10.1039/d0lc01013h
A 3-dimensional microfluidic platform for modeling human extravillous trophoblast invasion and toxicological screening
Abstract
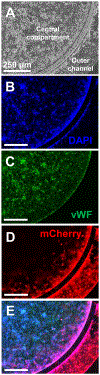
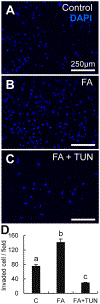
Placental trophoblast cells invasion into the maternal uterus is an essential and complex event in the formation of the maternal-fetal interface. Commonly used two-dimensional (2D) cell invasion tools do not accurately represent the in vivo cell invasion microenvironment. Three-dimensional (3D) silicone polymer polydimethylsiloxane (PDMS) microfluidic platforms are an emerging technology in developing organ-on-a-chip models. Here, we present a placenta-on-a-chip platform that enables the evaluation of trophoblast invasion with intraluminal flow within an engineered PDMS 3D microfluidic chip. This platform reproduces key elements of the placental microenvironment, including endothelial and trophoblast cells, layered with an extracellular matrix, and incorporates dynamic medium flow while allowing for real-time monitoring, imaging, evaluation of trophoblast cell invasion, and heterocellular cell-to-cell interactions. Coupled with fluorescent cell tagging and flow cytometry, this platform also allows collection of the invasive cells. This will help our understanding of pathways that regulate trophoblast cell invasion and may prove important for toxicological screening of exposures that interfere with invasiveness in a complex organ such as the placenta.
Conflict of interest statement
Figures



Similar articles
-
A Three-Dimensional Trophoblast Invasion Microfluidic Platform for Toxicological Screening.Methods Mol Biol. 2024;2728:223-234. doi: 10.1007/978-1-0716-3495-0_18. Methods Mol Biol. 2024. PMID: 38019404 Free PMC article.
-
A microfluidics assay to study invasion of human placental trophoblast cells.J R Soc Interface. 2017 May;14(130):20170131. doi: 10.1098/rsif.2017.0131. J R Soc Interface. 2017. PMID: 28566515 Free PMC article.
-
A Microphysiological Model to Mimic the Placental Remodeling during Early Stage of Pregnancy under Hypoxia-Induced Trophoblast Invasion.Biomimetics (Basel). 2024 May 12;9(5):289. doi: 10.3390/biomimetics9050289. Biomimetics (Basel). 2024. PMID: 38786499 Free PMC article.
-
Kisspeptins and the placenta: regulation of trophoblast invasion.Rev Endocr Metab Disord. 2007 Mar;8(1):31-9. doi: 10.1007/s11154-007-9030-8. Rev Endocr Metab Disord. 2007. PMID: 17351756 Review.
-
Human trophoblast invasion: new and unexpected routes and functions.Histochem Cell Biol. 2018 Oct;150(4):361-370. doi: 10.1007/s00418-018-1699-0. Epub 2018 Jul 26. Histochem Cell Biol. 2018. PMID: 30046889 Free PMC article. Review.
Cited by
-
Modeling development using microfluidics: bridging gaps to foster fundamental and translational research.Curr Opin Genet Dev. 2023 Oct;82:102097. doi: 10.1016/j.gde.2023.102097. Epub 2023 Aug 11. Curr Opin Genet Dev. 2023. PMID: 37573835 Free PMC article. Review.
-
Autophagy in reproduction and pregnancy-associated diseases.iScience. 2024 Oct 28;27(12):111268. doi: 10.1016/j.isci.2024.111268. eCollection 2024 Dec 20. iScience. 2024. PMID: 39628569 Free PMC article. Review.
-
Breakthroughs and Applications of Organ-on-a-Chip Technology.Cells. 2022 Jun 2;11(11):1828. doi: 10.3390/cells11111828. Cells. 2022. PMID: 35681523 Free PMC article. Review.
-
A Three-Dimensional Trophoblast Invasion Microfluidic Platform for Toxicological Screening.Methods Mol Biol. 2024;2728:223-234. doi: 10.1007/978-1-0716-3495-0_18. Methods Mol Biol. 2024. PMID: 38019404 Free PMC article.
-
Chemical mixture that targets the epidermal growth factor pathway impairs human trophoblast cell functions.Toxicol Appl Pharmacol. 2024 Feb;483:116804. doi: 10.1016/j.taap.2024.116804. Epub 2024 Jan 6. Toxicol Appl Pharmacol. 2024. PMID: 38185387 Free PMC article.
References
-
- Abou-Kheir W, Barrak J, Hadadeh O, Daoud G. 2017. Htr-8/svneo cell line contains a mixed population of cells. Placenta 50:1–7. - PubMed
-
- Ahmed T, Fellus I, Gaudet J, MacFarlane AJ, Fontaine-Bisson B, Bainbridge SA. 2016. Effect of folic acid on human trophoblast health and function in vitro. Placenta 37:7–15. - PubMed
-
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, et al. 1987. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47:3239–3245. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

