(Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus
- PMID: 33166419
- PMCID: PMC8095010
- DOI: 10.1002/14651858.CD005613.pub4
(Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus
Abstract
Background: Evidence that antihyperglycaemic therapy is beneficial for people with type 2 diabetes mellitus is conflicting. While the United Kingdom Prospective Diabetes Study (UKPDS) found tighter glycaemic control to be positive, other studies, such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, found the effects of an intensive therapy to lower blood glucose to near normal levels to be more harmful than beneficial. Study results also showed different effects for different antihyperglycaemic drugs, regardless of the achieved blood glucose levels. In consequence, firm conclusions on the effect of interventions on patient-relevant outcomes cannot be drawn from the effect of these interventions on blood glucose concentration alone. In theory, the use of newer insulin analogues may result in fewer macrovascular and microvascular events.
Objectives: To compare the effects of long-term treatment with (ultra-)long-acting insulin analogues (insulin glargine U100 and U300, insulin detemir and insulin degludec) with NPH (neutral protamine Hagedorn) insulin (human isophane insulin) in adults with type 2 diabetes mellitus.
Search methods: For this Cochrane Review update, we searched CENTRAL, MEDLINE, Embase, ICTRP Search Portal and ClinicalTrials.gov. The date of the last search was 5 November 2019, except Embase which was last searched 26 January 2017. We applied no language restrictions.
Selection criteria: We included randomised controlled trials (RCTs) comparing the effects of treatment with (ultra-)long-acting insulin analogues to NPH in adults with type 2 diabetes mellitus.
Data collection and analysis: Two review authors independently selected trials, assessed risk of bias, extracted data and evaluated the overall certainty of the evidence using GRADE. Trials were pooled using random-effects meta-analyses.
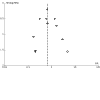
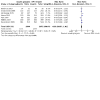
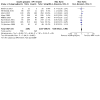
Main results: We identified 24 RCTs. Of these, 16 trials compared insulin glargine to NPH insulin and eight trials compared insulin detemir to NPH insulin. In these trials, 3419 people with type 2 diabetes mellitus were randomised to insulin glargine and 1321 people to insulin detemir. The duration of the included trials ranged from 24 weeks to five years. For studies, comparing insulin glargine to NPH insulin, target values ranged from 4.0 mmol/L to 7.8 mmol/L (72 mg/dL to 140 mg/dL) for fasting blood glucose (FBG), from 4.4 mmol/L to 6.6 mmol/L (80 mg/dL to 120 mg/dL) for nocturnal blood glucose and less than 10 mmol/L (180 mg/dL) for postprandial blood glucose, when applicable. Blood glucose and glycosylated haemoglobin A1c (HbA1c) target values for studies comparing insulin detemir to NPH insulin ranged from 4.0 mmol/L to 7.0 mmol/L (72 mg/dL to 126 mg/dL) for FBG, less than 6.7 mmol/L (120 mg/dL) to less than 10 mmol/L (180 mg/dL) for postprandial blood glucose, 4.0 mmol/L to 7.0 mmol/L (72 mg/dL to 126 mg/dL) for nocturnal blood glucose and 5.8% to less than 6.4% HbA1c, when applicable. All trials had an unclear or high risk of bias for several risk of bias domains. Overall, insulin glargine and insulin detemir resulted in fewer participants experiencing hypoglycaemia when compared with NPH insulin. Changes in HbA1c were comparable for long-acting insulin analogues and NPH insulin. Insulin glargine compared to NPH insulin had a risk ratio (RR) for severe hypoglycaemia of 0.68 (95% confidence interval (CI) 0.46 to 1.01; P = 0.06; absolute risk reduction (ARR) -1.2%, 95% CI -2.0 to 0; 14 trials, 6164 participants; very low-certainty evidence). The RR for serious hypoglycaemia was 0.75 (95% CI 0.52 to 1.09; P = 0.13; ARR -0.7%, 95% CI -1.3 to 0.2; 10 trials, 4685 participants; low-certainty evidence). Treatment with insulin glargine reduced the incidence of confirmed hypoglycaemia and confirmed nocturnal hypoglycaemia. Treatment with insulin detemir compared to NPH insulin found an RR for severe hypoglycaemia of 0.45 (95% CI 0.17 to 1.20; P = 0.11; ARR -0.9%, 95% CI -1.4 to 0.4; 5 trials, 1804 participants; very low-certainty evidence). The Peto odds ratio for serious hypoglycaemia was 0.16, 95% CI 0.04 to 0.61; P = 0.007; ARR -0.9%, 95% CI -1.1 to -0.4; 5 trials, 1777 participants; low-certainty evidence). Treatment with detemir also reduced the incidence of confirmed hypoglycaemia and confirmed nocturnal hypoglycaemia. Information on patient-relevant outcomes such as death from any cause, diabetes-related complications, health-related quality of life and socioeconomic effects was insufficient or lacking in almost all included trials. For those outcomes for which some data were available, there were no meaningful differences between treatment with glargine or detemir and treatment with NPH. There was no clear difference between insulin-analogues and NPH insulin in terms of weight gain. The incidence of adverse events was comparable for people treated with glargine or detemir, and people treated with NPH. We found no trials comparing ultra-long-acting insulin glargine U300 or insulin degludec with NPH insulin.
Authors' conclusions: While the effects on HbA1c were comparable, treatment with insulin glargine and insulin detemir resulted in fewer participants experiencing hypoglycaemia when compared with NPH insulin. Treatment with insulin detemir also reduced the incidence of serious hypoglycaemia. However, serious hypoglycaemic events were rare and the absolute risk reducing effect was low. Approximately one in 100 people treated with insulin detemir instead of NPH insulin benefited. In the studies, low blood glucose and HbA1c targets, corresponding to near normal or even non-diabetic blood glucose levels, were set. Therefore, results from the studies are only applicable to people in whom such low blood glucose concentrations are targeted. However, current guidelines recommend less-intensive blood glucose lowering for most people with type 2 diabetes in daily practice (e.g. people with cardiovascular diseases, a long history of type 2 diabetes, who are susceptible to hypoglycaemia or older people). Additionally, low-certainty evidence and trial designs that did not conform with current clinical practice meant it remains unclear if the same effects will be observed in daily clinical practice. Most trials did not report patient-relevant outcomes.
Trial registration: ClinicalTrials.gov NCT02451917 NCT00504673 NCT00941369 NCT00604396 NCT00949442 NCT00686712 NCT00604344 NCT00604253 NCT00687453 NCT00506662 NCT00795600 NCT00653341 NCT00174824 NCT02967211 NCT02967224 NCT00788840 NCT01310452 NCT01500850 NCT03389490.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
KH: was involved in the preparation of the report on long‐acting insulin analogues for the treatment of type 2 diabetes mellitus for the Institute for Quality and Efficiency in Health Care (
JE: none.
TS: none.
KJ: was involved in the preparation of the report on long‐acting insulin analogues for the treatment of type 2 diabetes mellitus for the Institute for Quality and Efficiency in Health Care (
AB: none.
AS: was involved in the preparation of the report on long‐acting insulin analogues for the treatment of type 2 diabetes mellitus for the Institute for Quality and Efficiency in Health Care (
Figures



























Update of
-
Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus.Cochrane Database Syst Rev. 2007 Apr 18;(2):CD005613. doi: 10.1002/14651858.CD005613.pub3. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2020 Nov 9;11:CD005613. doi: 10.1002/14651858.CD005613.pub4. PMID: 17443605 Updated.
References
References to studies included in this review
Berard 2015 {published data only}
-
- Berard L, Cameron B, Woo V, Stewart J. Replacing insulin glargine with neutral protamine Hagedorn (NPH) insulin in a subpopulation of study subjects in the action to control cardiovascular risk in diabetes (ACCORD): effects on blood glucose levels, hypoglycemia and patient satisfaction. Canadian Journal of Diabetes 2015;39(4):296-301. [PMID: ] - PubMed
-
- Berard LD, Woo VC. Safety and efficacy of NPH after switching from insulin glargine in Canadian patients. Diabetologia 2011;54:S430. [EMBASE: 70563203]
Betônico 2019 {published data only}
-
- Betônico CC, Titan SM, Correa-Giannella ML, Nery M, Queiroz M. Comparison of insulin glargine vs. NPH insulin in basal-bolus therapies in T2DM patients and CKD: preliminary results of a prospective, randomized, open-label trial. Diabetes 2016;65:A144-5. [CENTRAL: CN-01450000]
-
- Betônico CC, Titan SM, Lira A, Pelaes TS, Correa-Giannella ML, Nery M, et al. Insulin glargine U100 improved glycemic control and reduced nocturnal hypoglycemia in patients with type 2 diabetes mellitus and chronic kidney disease stages 3 and 4. Clinical Therapeutics 2019;41(10):2008-20. [PMID: ] - PubMed
-
- NCT02451917. Subcutaneous insulin glargine versus NPH insulin in patients with chronic kidney disease stages III and IV: randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02451917 (first received 22 May 2015).
Eliaschewitz 2006 {published data only}
-
- Eliaschewitz FG, Calvo C, Valbuena H, Ruiz M, Aschner P, Villena J, et al. Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Archives of Medical Research 2006;37(4):495-501. [PMID: ] - PubMed
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
Fajardo Montañana 2008 {published data only}
-
- EUCTR2005-000976-42. A multi-centre, open-labelled, randomised, two-group parallel, treat-to-target trial comparing the weight change in overweight and obese subjects with type 2 diabetes after 26 weeks of treatment with insulin detemir once daily versus insulin NPH once daily, both with insulin aspart in the mealtime (PREDICTIVE-BMI study) – PREDICTIVE-BMI study. www.clinicaltrialsregister.eu/ctr-search/trial/2005-000976-42/ES (first received 17 May 2006).
-
- Fajardo Montañana C, Hernandez Herrero C, Rivas Fernandez M. Less weight gain and hypoglycaemia with once-daily insulin detemir than NPH insulin in intensification of insulin therapy in overweight type 2 diabetes patients: the PREDICTIVE BMI clinical trial. Diabetic Medicine 2008;25(8):916-23. [PMID: ] - PubMed
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- NCT00504673. Comparison of insulin detemir versus insulin NPH on weight change in overweight and obese with type 2 diabetes (PREDICTIVE). clinicaltrials.gov/ct2/show/NCT00504673 (first received 20 July 2007).
-
- Novo Nordisk A/S. A multi-centre, open-labelled, randomized, two-group parallel, treat-to-target trial comparing the weight change in overweight and obese subjects with type 2 diabetes after 26 weeks of treatment with insulin detemir once daily versus insulin NPH once daily, both with insulin aspart in the mealtime. (PREDICTIVE-BMI study). http://filehosting.pharmacm.com/DownloadService.ashx?client=CTR_NVO_7271... (last accessed 3 November 2020).
Fritsche 2003 {published data only}
-
- Fritsche A, Schweitzer MA, Haring HU, Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine Hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Annals of Internal Medicine 2003;138(12):952-9. [PMID: ] - PubMed
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
Haak 2005 {published data only}
-
- Haak T, Tiengo A, Draeger E, Suntum M, Waldhausl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes, Obesity & Metabolism 2005;7(1):56-64. [PMID: ] - PubMed
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
Hermanns 2015 {published data only}
-
- EUCTR2009-010913-59. Health assessment, patient treatment satisfaction and quality-of-life in insulin-naive type 2 diabetes patients uncontrolled on OHA treatment initiating basal insulin therapy with either insulin glargine or NPH insulin added to therapies with oral hypoglycemic agents (HAPPY) (LANTU_L_04079 trial report). www.clinicaltrialsregister.eu/ctr-search/trial/2009-010913-59/results (last accessed 3 May 2017).
-
- Hermanns N, Kulzer B, Kohlmann T, Jacob S, Landgraf W, Theobald K, et al. Treatment satisfaction and quality-of-life between type 2 diabetes patients initiating long- vs intermediate-acting basal insulin therapy in combination with oral hypoglycemic agents – a randomized, prospective, crossover, open clinical trial. Health and Quality of Life Outcomes 2015;13:77. [PMID: ] - PMC - PubMed
-
- NCT00941369. Health assessment, patient treatment satisfaction and quality-of-life in insulin-naive type 2 diabetes patients uncontrolled on oral hypoglycemic agent treatment initiating basal insulin therapy with either insulin glargine or NPH insulin. clinicaltrials.gov/ct2/show/NCT00941369 (first received 17 July 2009).
Hermansen 2006 {published data only}
-
- Dailey G, Strange P, Riddle M. Reconsideration of severe hypoglycemic events in the treat-to-target trial. Diabetes Technology & Therapeutics 2009;11(8):477-9. [PMID: ] - PubMed
-
- Davies MJ, Derezinski T, Pedersen CB, Clauson P. Reduced weight gain with insulin detemir compared to NPH insulin is not explained by a reduction in hypoglycemia. Diabetes Technology & Therapeutics 2008;10(4):273-7. [PMID: ] - PubMed
-
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. Erratum: A 26-week randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people wih type 2 diabetes (Diabetes Care (2006) 29, (1269-1274)). Diabetes Care 2007;30(4):1035. [EMBASE: 46556609] - PubMed
-
- Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29(6):1269-74. [PMID: ] - PubMed
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
Home 2015 {published data only}
-
- EUCTR2007-006640-22. Superiority of insulin glargine Lantus vs NPH: "Treat to normoglycemia concept". Effect of insulin glargine in comparison to insulin NPH in insulin-naïve people with type 2 diabetes mellitus treated with at least one OAD and not adequately controlled (LANCELOT) (LANTU_C_02762 trial report). www.clinicaltrialsregister.eu/ctr-search/trial/2007-006640-22/results (last accessed 19 July 2017).
-
- Home PD, Bolli GB, Mathieu C, Deerochanawong C, Landgraf W, Candelas C, et al. Modulation of insulin dose titration using a hypoglycaemia-sensitive algorithm: insulin glargine versus neutral protamine Hagedorn insulin in insulin-naive people with type 2 diabetes. Diabetes, Obesity & Metabolism 2015;17(1):15-22. [PMID: ] - PMC - PubMed
-
- NCT00949442. Lantus versus NPH: comparison in insulin naive people not adequately controlled with at least one oral anti diabetics (OAD) treatment (LANCELOT). clinicaltrials.gov/ct2/show/NCT00949442 (first received 30 July 2009).
Hsia 2011 {published data only}
-
- Hsia SH. Comparison of single-dose regimens of insulin glargine versus NPH among inner city ethnic minority type 2 diabetic patients uncontrolled on oral agents. Journal of Investigative Medicine 2010;58(1):136. [EMBASE: 70981533]
-
- NCT00686712. Insulin glargine at bedtime or in AM versus NPH. clinicaltrials.gov/ct2/show/NCT00686712 (first received 7 October 2010).
Kawamori 2003 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Kawamori R, Iwamoto Y, Kadowaki T, Iwasaki M. Efficacy and safety of insulin glargine in concurrent use with oral hypoglycemic agents for the treatment of type 2 diabetes patients. Rinshoiyaku 2003;19(5):445-64.
Kobayashi 2007 A {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Ishii H, Iwamoto Y, Kaku K, Kawamori R, Kobayashi M, Tajima N. Night-time QOL and treatment satisfaction in diabetes mellitus patients treated with insulin detemir used in basal-bolus therapy – randomized multicenter open-label parallel group phase 3 trial compared to NPH insulin. Journal of the Japan Diabetes Society 2009;52(8):691-701. [DOI: 10.11213/tonyobyo.52.691] - DOI
-
- Kobayashi M, Iwamoto Y, Kaku K, Kawamori R, Tajima N. Phase 3 clinical trial of insulin detemir for patients with insulin-dependent diabetes during basal-bolus therapy – a study of efficacy and safety with NPH insulin as control. Journal of the Japan Diabetes Society 2007;50(9):649-63. [DOI: 10.11213/tonyobyo.50.649] - DOI
-
- NCT00604344. Efficacy and the safety of insulin detemir in subjects with insulin requiring diabetes. clinicaltrials.gov/ct2/show/NCT00604344 (first received 30 January 2008).
-
- Novo Nordisk A/S. A 48-week, randomised, multi-centre, open-labelled, parallel-group trial to compare the efficacy and the safety of NN304 (insulin detemir) and NPH human insulin in subjects with insulin requiring diabetes mellitus on a basal-bolus regimen. https://s3.amazonaws.com/ctr-nvo-7271/NN304-1476/aa9b4da3-1f92-460d-af0a... (last accessed 3 November 2020).
Kobayashi 2007 B {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Kobayashi M, Iwamoto Y, Kaku K, Kawamori R, Tajima N. Phase 3 clinical trial of insulin detemir for type 2 diabetes patients with insufficient blood sugar control in oral diabetes drug therapy. Journal of the Japan Diabetes Society 2007;50(9):665-77. [DOI: 10.11213/tonyobyo.50.665] - DOI
-
- NCT00604253. Efficacy and safety of insulin detemir in type 2 diabetes inadequately controlled on OHA therapy alone. www.clinicaltrials.gov/ct2/show/results/NCT00604253 (first received 30 January 2008).
-
- Novo Nordisk A/S. A 36-week, randomised, multi-centre, open-labelled, parallel-group trial to compare the efficacy and the safety of NN304 and NPH human insulin once daily treatment as add-on to current OHA therapy in subjects with type 2 diabetes mellitus who are inadequately controlled on OHA therapy. http://filehosting.pharmacm.com/DownloadService.ashx?client=CTR_NVO_7271... (last accessed 3 November 2020).
Massi 2003 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Massi BM, Humburg E, Dressler A, Ziemen M. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Hormone & Metabolic Research 2003;35(3):189-96. [PMID: ] - PubMed
-
- Yki-Jarvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 2000;23(8):1130-6. [EMBASE: 2006092604] - PubMed
NCT00687453 {published data only}
-
- NCT00687453. The utility of insulin glargine (Lantus) compared to NPH in ethnic minority type 2 diabetic subjects on combination insulin-oral agent therapy. clinicaltrials.gov/ct2/show/NCT00687453 (first received 30 May 2008).
NN304‐1337 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
NN304‐1808 {published data only}
-
- Doucet J, Le Floch J, Hanaire H, Courreges J, Alexandre B, Bauduceau B. Comparison of safety between detemir and neutral protamine Hagedorn (NPH) in ageing subjects: the '3 L study'. Fundamental and Clinical Pharmacology 2011;25:82. [EMBASE: 70653089]
-
- EUCTR2006-006589-41-FR. Levemir® in ageing patients with type 2 diabetes A seven-month open-labelled randomised multi-centre two-group parallel trial comparing administration of insulin detemir once daily in the morning versus NPH insulin once daily in the morning in ageing subjects with type 2 diabetes. The 3L Study (Levemir in Late Life) Trial Phase: 4. www.clinicaltrialsregister.eu/ctr-search/trial/2006-006589-41/FR (first received 27 March 2007).
-
- NCT00506662. The effect of insulin detemir on glucose control in ageing subjects with type 2 diabetes. clinicaltrials.gov/ct2/show/study/NCT00506662 (first received 25 July 2007).
-
- Novo Nordisk A/S. A seven-month, open-labelled, randomised, multicentre, two-group, parallel trial comparing administration of insulin detemir once daily in the morning versus NPH insulin once daily in ageing patients with type 2 diabetes: The 3L Study (Levemir in Later Life) (NN304-1808 Clinical Trial Report). http://filehosting.pharmacm.com/DownloadService.ashx?client=CTR_NVO_7271... (last accessed 3 November 2020).
NN304‐3614 {published data only}
-
- EUCTR2008-003739-19. Ensayo multicéntrico, abierto, aleatorizado, de dos grupos, paralelo para comparar el cambio en la distribución de grasa en sujetos con sobrepeso y obesos con diabetes tipo 2 tras 26 semanas de tratamiento con insulina detemir una vez al día frente a insulina NPH una vez al día, ambas con insulina aspart a la hora de las comidas. www.clinicaltrialsregister.eu/ctr-search/trial/2008-003739-19/ES (first received 19 September 2008).
-
- NCT00795600. A multi-centre, open-labelled, randomised, two-group parallel trial comparing the change in fat distribution in overweight and obese subjects with type 2 diabetes after 26 weeks of treatment with insulin detemir once daily versus insulin NPH once daily, both with insulin apart at mealtimes. clinicaltrials.gov/ct2/show/NCT00795600 (first received 21 November 2008).
-
- Novo Nordisk A/S. A multi-centre, open-labelled, randomised, two-group parallel trial comparing the change in fat distribution in overweight and obese subjects with type 2 diabetes after 26 weeks of treatment with insulin detemir once daily versus insulin NPH once daily, both with insulin aspart at mealtimes (NN304-3614 Clinical Trial Report). http://filehosting.pharmacm.com/DownloadService.ashx?client=CTR_NVO_7271... (last accessed 3 November 2020).
-
- Tinahones FJ, Gomez-Huelgas R, Garcia-Alegria J, Tapia-Guerrero M, Martos-Perez F. Fat distribution, HbA1c and hypoglycaemia in overweight and obese patients with type 2 diabetes: a comparison of NPH and insulin detemir from a pilot randomised clinical trial. Diabetologia 2011;54:S273. [EMBASE: 70562816]
-
- Tinahones FJ, Gomez-Huelgas R, Garciaalegria J, Tapia-Guerrero MJ, Perez FM. The effect of insulin detemir on fat distribution and weight parameters in overweight and obese subjects with type 2 diabetes. Diabetes 2011;60:A309. [EMBASE: 70628891]
Pan 2007 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Pan CY, Sinnassamy P, Chung KD, Kim KW, Group LS . Insulin glargine versus NPH insulin therapy in Asian type 2 diabetes patients. Diabetes Research & Clinical Practice 2007;76(1):111-8. [PMID: ] - PubMed
Riddle 2003 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- NCT00653341. Effect of insulin glargine in insulin naïve subjects with type 2 diabetes on oral hypoglycemic agent(s). www.clinicaltrials.gov/ct2/show/results/NCT00653341 (first received 4 April 2008).
-
- Riddle MC, Rosenstock J, Gerich J, Insulin G. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26(11):3080-6. [PMID: ] - PubMed
Rosenstock 2001 {published data only}
-
- Fonseca V, Bell DS, Berger S, Thomson S, Mecca TE. A comparison of bedtime insulin glargine with bedtime neutral protamine Hagedorn insulin in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study. American Journal of the Medical Sciences 2004;328(5):274-80. [PMID: ] - PubMed
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24(4):631-6. [PMID: ] - PubMed
Rosenstock 2009 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- NCT00174824. Comparison of insulin glargine and NPH human insulin in progression of diabetic retinopathy in type 2 diabetic patients. clinicaltrials.gov/ct2/show/study/NCT00174824 (first received 15 September 2005).
-
- Rosenstock J, Fonseca V, Dain MP, Riddle M. Estimating number-needed-to-treat to avoid hypoglycaemic episodes in people with type 2 diabetes: a post-hoc analysis of a prospective randomized controlled trial comparing once-daily insulin glargine with twice-daily NPH insulin. Canadian Journal of Diabetes 2009;33(3):185-6. [EMBASE: 70031352]
-
- Rosenstock J, Fonseca V, McGill JB, Riddle M, Halle JP, Hramiak I, et al. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: a long-term, randomised, open-label study. Diabetologia 2009;52(9):1778-88. [PMID: ] - PMC - PubMed
-
- Rosenstock J, Fonseca V, McGill JB, Riddle M, Halle JP, Hramiak I, et al. Similar risk of malignancy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia 2009;52(9):1971-3. [PMID: ] - PMC - PubMed
Yki‐Järvinen 2006 {published data only}
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
-
- Varewijck AJ, Janssen JA, Vahatalo M, Lamberts SW, Ki-Jarvinen HY. IGF-I bioactivity decreases during insulin therapy in type 2 diabetic patients: comparison of high doses of insulin glargine and NPH insulin in the LANMET study. In: Endocrine Reviews Conference: 93rd Annual Meeting and Expo of the Endocrine Society, ENDO. Vol. 32 (3 Meeting Abstracts). 2011. [EMBASE: 70676865]
-
- Varewijck AJ, Yki-Jarvinen H, Schmidt R, Tennagels N, Janssen JA. Concentrations of insulin glargine and its metabolites during long-term insulin therapy in type 2 diabetic patients and comparison of effects of insulin glargine, its metabolites, IGF-I, and human insulin on insulin and IGF-I receptor signaling. Diabetes 2013;62(7):2539-44. [PMID: ] - PMC - PubMed
-
- Varewijck AJ, Yki-Jarvinen H, Schmidt R, Tennagels N, Janssen JAMJL. Comparison of effects of insulin glargine, its metabolites, IGF-I and human insulin on insulin-and IGF-I receptor signaling in type 2 diabetic patients. In: Endocrine Reviews Conference: 95th Annual Meeting and Expo of the Endocrine Society, ENDO. Vol. 34 (3 suppl 1). 2013. [EMBASE: 71784187] - PMC - PubMed
Yokoyama 2006 {published data only}
-
- Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Research & Clinical Practice 2006;73(1):35-40. [PMID: ] - PubMed
References to studies excluded from this review
Bellido 2014 {published data only}
-
- Bellido V, Suarez L, Rodriguez MG, Sanchez C, Dieguez M, Riestra M, et al. Comparison of insulin regimens with basal bolus vs mixed insulin in hospitalized patients with hyperglycemia. Diabetes 2014;63:A239-40. [EMBASE: 71559325]
Bi 2012 {published data only}
-
- Bi Y, Li X, Yang D, Hao Y, Liang H, Zhu D, et al. Comparative efficacy and safety of long-acting insulin analogs in patients with type 2 diabetes failing on oral therapy: systemic review and meta-analyses. Journal of Diabetes Investigation 2012;3(3):283-93. [EMBASE: 364970679] [PMID: ] - PMC - PubMed
Bolli 2012 {published data only}
-
- Bolli GB, Porcellati F, Lin J, Cali A, Wang E, Dain MP, et al. Reduction in HbA1c and hypoglycaemia rates with insulin glargine vs NPH in treatment-naive patients with type 2 diabetes stratified by BMI: pooled analysis of 6 clinical trials. Diabetologia 2012;55:S385. [EMBASE: 70888959]
Currie 2009 {published data only}
-
- Currie CJ. The longest ever randomised controlled trial of insulin glargine: study design and HbA1c findings. Diabetologia 2009;52(10):2234-5. [CENTRAL: CN-00753653] - PubMed
Dailey 2013 {published data only}
-
- Dailey GE, Gao L, Aurand L, Garg SK. Impact of diabetes duration on hypoglycaemia in patients with type 2 diabetes treated with insulin glargine or NPH insulin. Diabetes, Obesity & Metabolism 2013;15(12):1085-92. [PMID: ] - PubMed
Freemantle 2016 {published data only}
Freemantle 2020a {published data only}
-
- EUCTR2015-001832-39-IE. A twenty-six week, randomized, open-label, 2-arm parallel group real world pragmatic trial to assess the clinical and health outcomes benefit of transition to Toujeo compared to standard of care insulin, in basal insulin treated patients with uncontrolled type 2 diabetes mellitus, with six month extension. www.clinicaltrialsregister.eu/ctr-search/trial/2015-001832-39/IE (first received 13 August 2015).
-
- Freemantle N, Mauricio D, Giaccari A, Bailey T, Roussel R, Franco D, et al. Real-world outcomes of treatment with insulin glargine 300 U/mL versus standard-of-care in people with uncontrolled type 2 diabetes mellitus. Current Medical Research and Opinion 2020;36(4):571-81. [PMID: ] - PubMed
-
- NCT02967211. A twenty-six week, randomized, open-label, 2-arm parallel group real world pragmatic trial to assess the clinical and health outcomes benefit of transition to Toujeo compared to "standard of care" insulin in basal insulin treated patients with uncontrolled type 2 diabetes mellitus, with six-month extension. www.clinicaltrials.gov/ct2/show/record/NCT02967211 (first received 18 November 2016).
Freemantle 2020b {published data only}
-
- Freemantle N, Mauricio D, Giaccari A, Bailey T, Roussel R, Franco D, et al. Real-world outcomes of treatment with insulin glargine 300 U/mL versus standard-of-care in people with uncontrolled type 2 diabetes mellitus. Current Medical Research and Opinion 2020;36(4):571-81. [PMID: ] - PubMed
-
- NCT02967224. A twenty-six week, randomized, open-label, 2-arm parallel group real world pragmatic trial to assess the clinical and health outcomes benefit of Toujeo compared to "standard of care" insulin for initiating basal insulin in insulin naïve patients with uncontrolled type 2 diabetes mellitus, with 6-month extension. www.clinicaltrials.gov/ct2/show/record/NCT02967224 (first received 18 November 2016).
Frier 2013 {published data only}
-
- Frier BM, Russell-Jones D, Heise T. A comparison of insulin detemir and neutral protamine Hagedorn (isophane) insulin in the treatment of diabetes: a systematic review. Diabetes, Obesity & Metabolism 2013;15(11):978-86. [PMID: ] - PubMed
Fritsche 2010 {published data only}
-
- Fritsche A, Larbig M, Haring HU. A basal-bolus regimen of insulin glargine and insulin glulisine results in a lower rate of hypoglycaemia relative to endpoint HbA1c versus twice daily premixed insulin in type 2 diabetes patients. Diabetologia 2010;53:S232. [EMBASE: 70262613]
Hoogwerf 2016 {published data only}
ISRCTN76123473 {published data only}76123473
-
- ISRCTN76123473. Randomised controlled trial of nocturnal insulin glargine versus human insulatard in combination with metformin in patients with type two diabetes currently treated with metformin/insulatard combination therapy. www.isrctn.com/ISRCTN76123473 (first received 12 September 2003).
Jensen 2010 {published data only}
-
- Jensen MG, Hansen M, Brock B, Rungby J. Differences between long-acting insulins for the treatment of type 2 diabetes. Expert Opinion on Pharmacotherapy 2010;11(12):2027-35. [PMID: ] - PubMed
Jiang 2008 {published data only}
-
- Jiang JH, Cui JN. Comparison of the enhanced insulin treatments for newly-diagnosed type 2 diabetes. Chinese Journal of New Drugs 2008;17(11):968-70. [EMBASE: 352054989]
Johnson 2009 {published data only}
-
- Johnson CK, Shimshi M. When a unit of insulin is not a unit: detemir dosing and insulin cost in type 2 diabetes mellitus. Insulin 2009;4(2):87-93. [EMBASE: 354529395]
Leal 2008 {published data only}
-
- Leal S. Long-acting insulins for the treatment of type 2 diabetes. Pharmacy Times 2008;74(10):38-40. [EMBASE: 352685306]
Lin 2014 {published data only}
-
- Lin J, Lingohr-Smith M. Comparison of insulin glargine versus NPH insulin treatment among patients with type 2 diabetes based on review of clinical trial results. Value in Health 2014;17(3):A238. [EMBASE: 71488816]
Mu 2011 {published data only}
-
- Mu P, Lu H, Zhang G, Chen Y, Fu J, Wang M, et al. Comparison of fasting capillary glucose variability between insulin glargine and NPH. Diabetes Research & Clinical Practice 2011;91(1):e4-7. [PMID: ] - PubMed
NCT01854723 {published data only}
-
- NCT01854723. Basal insulin therapy in patients with insulin resistance: a 6 month comparison of insulin glargine and NPH insulin. www.clinicaltrials.gov/ct2/show/record/NCT01854723 (first received 15 May 2013).
Oster 2016 {published data only}
-
- Meneghini L, Sullivan S, Oster G, Busch R, Dauchy A, Perfetti R, et al. A randomized prospective pragmatic real-world clinical trial of insulin glargine 300 u/ml versus other basal insulins in insulin-naive patients with type 2 diabetes: a 6-month analysis of the achieve control study. Journal of Managed Care and Specialty Pharmacy 2018;24(10A):S18. [CENTRAL: CN-01670031]
-
- Oster G, Sullivan SD, Dalal MR, Kazemi MR, Rojeski M, Wysham CH, et al. Achieve control: a pragmatic clinical trial of insulin glargine 300 U/mL versus other basal insulins in insulin-naive patients with type 2 diabetes. Postgraduate Medicine 2016;128(8):731-9. [PMID: ] - PubMed
Owens 2014 {published data only}
-
- Owens DR, Trayor L, Landgraf W, Mullins P. New meta-analysis of patient-level data on efficacy and hypoglycaemia with insulin glargine or NPH insulin in type 2 diabetes mellitus (T2DM) according to concomitant oral therapy. Value in Health 2014;17(7):A335. [PMID: ] - PubMed
Owens 2017 {published data only}
-
- Owens DR, Traylor L, Mullins P, Landgraf W. Patient-level meta-analysis of efficacy and hypoglycaemia in people with type 2 diabetes initiating insulin glargine 100U/mL or neutral protamine Hagedorn insulin analysed according to concomitant oral antidiabetes therapy. Diabetes Research & Clinical Practice 2017;124:57-65. [PMID: ] - PubMed
Peterson 2006 {published data only}
-
- Peterson GE. Intermediate and long-acting insulins: a review of NPH insulin, insulin glargine and insulin detemir. Current Medical Research & Opinion 2006;22(12):2613-9. [PMID: ] - PubMed
Philis‐Tsimikas 2008 {published data only}
-
- Philis-Tsimikas A. An update on the use of insulin detemir, with a focus on type 2 diabetes (drug evaluation update). Expert Opinion on Pharmacotherapy 2008;9(12):2181-95. [PMID: ] - PubMed
Porcellati 2017 {published data only}
-
- Porcellati F, Lin J, Lucidi P, Bolli GB, Fanelli CG. Impact of patient and treatment characteristics on glycemic control and hypoglycemia in patients with type 2 diabetes initiated to insulin glargine or NPH: a post hoc, pooled, patient-level analysis of 6 randomized controlled trials. Medicine 2017;96(5):e6022. [PMID: ] - PMC - PubMed
Puig 2012 {published data only}
-
- Puig A, Prieto L, Alba D, Solano MP. Comparative trial between combination of glargine and lispro insulin versus NPH and regular insulin using a basal/bolus approach in hospitalized patients with type 2 diabetes. Diabetes 2012;61:A300. [EMBASE: 70797758]
Ramirez de Arellano 2014 {published data only}
-
- Ramirez de Arellano A, Morales C, De Luis D, Ferrario MG, Lizan L. Short-term cost-effectiveness analysis of insulin detemir versus insulin neutral protamine Hagedorn (NPH) in patients with type 2 diabetes mellitus in Spain. Value in Health 2014;17(7):A343. [EMBASE: 71672532] - PubMed
Rys 2015 {published data only}
-
- Rys P, Wojciechowski P, Rogoz-Sitek A, Niesyczynski G, Lis J, Syta A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetologica 2015;52(4):649-62. [PMID: ] - PMC - PubMed
Schwartz 2011 {published data only}
-
- Schwartz AR, Miniter C, Vasquez L, Giovanniello J, Vogel D. Titration of insulin using a telephone protocol in a veterans affairs primary care clinic. Journal of General Internal Medicine 2011;26:S560. [EMBASE: 70654333]
Smith 2008 {published data only}
-
- Smith SA, Hassan Murad M. Review: long-acting insulin analogues do not improve glycaemic control but reduce nocturnal hypoglycaemia. Evidence-Based Medicine 2008;13(3):79. [EMBASE: 351836801] - PubMed
Tamaki 2008 {published data only}
-
- Tamaki M, Shimizu T, Kanazawa A, Fujitani Y, Watada H, Kawamori R, et al. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Research & Clinical Practice 2008;81(2):e1-3. [PMID: ] - PubMed
Tilling 2011 {published data only}
-
- Tilling C, Vora J, Keech M. A cost comparison of a basal bolus regimen (insulin glargine and insulin glulisine) with a conventional pre-mixed insulin regimen in type-2 diabetes patients – the GINGER study. Value in Health 2011;14(3):A94. [EMBASE: 70490942]
Van Avendonk 2009 {published data only}
-
- Van Avendonk MJ, Rutten G. Insulin therapy in type 2 diabetes: what is the evidence? Diabetes, Obesity & Metabolism 2009;11(5):415-32. [CENTRAL: CN-00958921] - PubMed
Vora 2011 {published data only}
-
- Vora JP, Punekar YS, Keech ML. A cost comparison of a basal-bolus regimen (glargine and glulisine) with a premixed insulin regimen in type 2 diabetes patients: the GINGER study. British Journal of Diabetes and Vascular Disease 2011;11(6):314-8. [EMBASE: 364032493]
Wang 2015 {published data only}
-
- Wang H, Zhang Q, Frois C, Vlajnic A, Wu E, Gerrits C, et al. Safety and efficacy of insulin glargine 300 U/mL (GLA-300) compared with other basal insulin therapies in patients with type 2 diabetes mellitus (T2DM) – a network meta-analysis (NMA). Diabetes 2015;64:A26. [EMBASE: 71939926] - PMC - PubMed
Wojciechowski 2013 {published data only}
-
- Wojciechowski P, Rys P, Rogoz-Sitek A, Niesyczynski G, Syta A, Lis J, et al. Clinical efficacy and safety of insulin glargine as compared to other insulin preparations in the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetologia 2013;56:S419. [EMBASE: 71439464]
References to studies awaiting assessment
NCT00788840 {published data only}72730583
-
- EUCTR2007-003085-17-GB. The effect of insulin detemir on energy balance, postprandial nutrient handling, body fat distribution, and adipose tissue metabolism and gene expression in patients with type 2 diabetes. www.clinicaltrialsregister.eu/ctr-search/trial/2007-003085-17/GB (first received 26 November 2007).
-
- ISRCTN72730583. The effect of insulin detemir on energy balance, postprandial nutrient handling, body fat distribution and adipose tissue metabolism and gene expression in patients with type 2 diabetes. www.isrctn.com/ISRCTN72730583 (first received 30 July 2010).
-
- NCT00788840. A 24-week, national, single-centre, open-labelled, randomised, parallel-group trial comparing energy expenditure with insulin detemir versus NPH insulin using a vasal-volus regimen with insulin aspart as the mealtime insulin in subjects with type 2 diabetes. www.clinicaltrials.gov/ct2/show/study/NCT00788840 (first received 11 November 2008).
NCT01310452 {published data only}
-
- NCT01310452. A multi-centre, open-labeled, randomized, parallel study on liver fat content and visceral fat mass in overweight and obese type 2 diabetes patients after 26 weeks treatment with insulin detemir once daily versus insulin NPH once daily. www.clinicaltrials.gov/ct2/show/study/NCT01310452 (first received 8 March 2011).
NCT01500850 {published data only}
-
- NCT01500850. Establishing cardiovascular biomarkers to define preferred lantus use. www.clinicaltrials.gov/ct2/show/record/NCT01500850 (first received 29 December 2011).
References to ongoing studies
EUCTR2017‐004454‐42‐ES {published data only}
-
- EUCTR2017-004454-42-ES. An interventional study to arrest the progression of cognitive decline in diabetic patients at high risk of developing Alzheimer's disease by reducing hypoglycemic events (low blood glucose). www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:201... (first received 25 January 2018).
NCT03389490 {published data only}
-
- NCT03389490. A pilot study to describe the glycaemic variability of insulin glargine 300U/ml versus NPH (neutral protamine Hagedorn) in the insulin-naïve type 2 diabetes patients following a patient-adjusted insulin algorithm in Hong Kong. www.clinicaltrials.gov/ct2/show/NCT03389490 (first received 3 January 2018).
Additional references
ACCORD 2008
ADA 2005
-
- Workgroup on Hypoglycemia, American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28(5):1245-9. [PMID: ] - PubMed
ADA 2020
-
- American Diabetes Association. Introduction: standards of medical care in diabetes. Diabetes Care 2020;43:S1-212. - PubMed
Adler 1997
-
- Adler AI, Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Smith DG. Risk factors for diabetic peripheral sensory neuropathy. Results of the Seattle Prospective Diabetic Foot Study. Diabetes Care 1997;20(7):1162-7. [PMID: ] - PubMed
ADVANCE 2008
-
- Advance Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358:2560-72. [PMID: ] - PubMed
Ahrén 2013
Bell 2013
-
- Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psychooncology 2013;22:1738-47. [PMID: ] - PubMed
Borenstein 2017a
-
- Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I² is not an absolute measure of heterogeneity. Research Synthesis Methods 2017;8(1):5-18. - PubMed
Borenstein 2017b
-
- Borenstein M. Prediction intervals. www.meta-analysis.com/prediction (accessed 3 July 2017).
Boutron 2014
-
- Boutron I, Altman DG, Hopewell S, Vera-Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120-6. [PMID: ] - PubMed
Buch 2011
-
- Buch MH, Aletaha D, Emery P, Smolen JS. Reporting of long-term extension studies: lack of consistency calls for consensus. Annals of the Rheumatic Diseases 2011;70(6):886-90. [PMID: ] - PubMed
CONSORT
-
- The CONSORT statement. www.consort-statement.org (accessed 29 August 2017).
Cooper 2019
Corbett 2014
-
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79-85. [PMID: ] - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Duckworth 2009
-
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New England Journal of Medicine 2009;360:129-39. [PMID: ] - PubMed
EMA 2003
-
- European Public Assessment Report (EPAR) on Lantus (Insulin Glargine) – Scientific Discussion (2003). www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussi... (accessed 29 August 2017).
EMA 2004
-
- European Public Assessment Report (EPAR) on Levemir (Insulin Detemir) – Scientific Discussion (2004). www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussi... (accessed 29 August 2017).
EMA 2012
-
- Committee for Medicinal Products for Human Use (CHMP) assessment report on Tresiba (Insulin degludec) (2012). www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussi... (accessed 29 August 2017).
FDA 2000
-
- Center for Drug Evaluation and Research (CDER). Application Number 21-081: Lantus (Insulin Glargine) – Pharmacology Review(s) (2000). www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21081_Lantus_pharmr_P1.pdf and www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21081_Lantus_pharmr_P2.pdf (accessed 29 August 2017).
FDA 2005
-
- Center for Drug Evaluation and Research (CDER). Application Number 21-536: Levemir (Insulin Detemir) – Pharmacology Review(s) (2005). www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021-536_Levemir_pharmr.PDF (accessed 29 August 2017).
Gerstein 2012
-
- Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, et al (ORIGIN Trial Investigators). Basal insulin and cardiovascular and other outcomes in dysglycemia. New England Journal of Medicine 2012;367(4):319-28. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version 25 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. www.guidelinedevelopment.org.
Grant 1993
-
- Grant MB, Mames RN, Fitzgerald C, Ellis EA, Aboufriekha M, Guy J. Insulin-like growth factor I acts as an angiogenic agent in rabbit cornea and retina: comparative studies with basic fibroblast growth factor. Diabetologia 1993;36(4):282-91. [PMID: ] - PubMed
Hart 2012
Heinemann 2000
-
- Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000;23(5):644-9. [PMID: ] - PubMed
Hemkens 2009
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [PMID: ] - PubMed
Higgins 2003
Higgins 2009
Higgins 2019a
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
-
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Holman 2008
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine 2008;359:1577-89. [PMID: ] - PubMed
Hozo 2005
Hróbjartsson 2013
-
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201-11. [PMID: ] - PMC - PubMed
ICH 2016
-
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2). www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E... (accessed 29 August 2017).
IQWiG 2009
-
- Institute for Quality and Efficiency in Health Care (IQWiG). Long-acting insulin analogues in the treatment of diabetes mellitus type 2 (IQWiG Reports – Commission No. A05-03). Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), 2009.
Jones 2015
Jorgensen 1992
-
- Jorgensen LN, Didriksen LH, Drejer K. Cardinogen effect of the human insulin analogue B10 Asp in female rats. Diabetologia 1992;35:A3.
King 1985
Kirkham 2010
Klein 1995
-
- Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 1995;18(2):258-68. [PMID: ] - PubMed
Kooy 2009
-
- Kooy A, Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Archives of Internal Medicine 2009;169:616-25. [PMID: ] - PubMed
Kurtzhals 2000
-
- Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49(6):999-1005. [PMID: ] - PubMed
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Lepore 2000
-
- Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di VA, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 2000;49(12):2142-8. [PMID: ] - PubMed
Lexchin 2003
Liberati 2009
Lipska 2018
-
- Lipska KJ, Parker MM, Moffet HH, Huang ES, Karter AJ. Association of initiation of basal insulin analogs vs neutral protamine Hagedorn insulin with hypoglycemia-related emergency department visits or hospital admissions and with glycemic control in patients with type 2 diabetes. JAMA 2018;320(1):53-62. [PMID: ] - PMC - PubMed
Madenidou 2018
-
- Madenidou AV, Paschos P, Karagiannis T, Katsoula A, Athanasiadou E, Kitsios K, et al. Comparative benefits and harms of basal insulin analogues for type 2 diabetes: a systematic review and network meta-analysis. Annals of Internal Medicine 2018;169(3):165-74. [PMID: ] - PubMed
Marso 2016
Mathieu 2009
-
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. [PMID: ] - PubMed
Meader 2014
Megan 2012
-
- Megan B, Pickering RM, Weatherall M. Design, objectives, execution and reporting of published open-label extension studies. Journal of Evaluation in Clinical Practice 2012;18(2):209-15. [PMID: ] - PubMed
Mühlhauser 1998
-
- Mühlhauser I, Overmann H, Bender R, Bott U, Berger M. Risk factors of severe hypoglycaemia in adult patients with type 1 diabetes – a prospective population based study. Diabetologia 1998;41:1274-82. [PMID: ] - PubMed
Review Manager 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Riley 2011
-
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [PMID: ] - PubMed
Rosenstock 2018
-
- Rosenstock J, Cheng A, Ritzel R, Bosnyak Z, Devisme C, Cali AM, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care 2018;41(10):2147-54. [PMID: ] - PubMed
Scherer 2018
Schroll 2015
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the confidence in or quality of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Singh 2007
-
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 2007;298:1189-95. [PMID: ] - PubMed
Sterne 2011
-
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [PMID: ] - PubMed
Turner 1998
UKPDS‐33 1998
-
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53. [PMID: ] - PubMed
UKPDS 34 1998
-
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352(9131):854-65. [PMID: ] - PubMed
Wong 2006
Zinman 2015
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine 2015;373:2117-28. [PMID: ] - PubMed
References to other published versions of this review
Horvath 2007
-
- Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No: CD005613. [DOI: 10.1002/14651858.CD005613.pub3] - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

