Full range of population Eliciting Dose values for 14 priority allergenic foods and recommendations for use in risk characterization
- PMID: 33166672
- PMCID: PMC7864389
- DOI: 10.1016/j.fct.2020.111831
Full range of population Eliciting Dose values for 14 priority allergenic foods and recommendations for use in risk characterization
Abstract
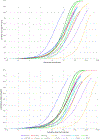
Previously, we published selected Eliciting Dose (ED) values (i.e. ED01 and ED05 values) for 14 allergenic foods, predicted to elicit objective allergic symptoms in 1% and 5%, respectively, of the allergic population (Remington et al., 2020). These ED01 and ED05 values were specifically presented and discussed in the context of establishing Reference Doses for allergen management and the calculation of Action Levels for Precautionary Allergen Labeling (PAL). In the current paper, we publish the full range of ED values for these allergenic foods and provide recommendations for their use, specifically in the context of characterizing risks of concentrations of (unintended) allergenic proteins in food products. The data provided in this publication give risk assessors access to full population ED distribution information for 14 priority allergenic foods, based on the largest threshold database worldwide. The ED distributions were established using broad international consensus regarding suitable datapoints and methods for establishing individual patient's NOAELs and LOAELs and state of the art statistical modelling. Access to these ED data enables risk assessors to use this information for state-of-the-art food allergen risk assessment. This paper contributes to a harmonization of food allergen risk assessment and risk management and PAL practices.
Keywords: Allergenic food; Eliciting dose; Food allergen; Food allergy; Risk assessment; Risk management.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Allen KJ, Remington BC, Baumert JL, Crevel RWR, Houben GF, Brooke-Taylor S, Kruizinga AG, Taylor SL Allergen reference doses for precautionary labeling (VITAL 2.0): Clinical implications (2014) Journal of Allergy and Clinical Immunology, 133 (1), pp. 156–164. 10.1016/j.jaci.2013.06.042 - DOI - PubMed
-
- Blom WM, Michelsen-Huisman AD, van Os-Medendorp H, van Duijn G, de Zeeuw-Brouwer M-L, Versluis A, Castenmiller JJM, Noteborn HPJM, Kruizinga AG, Knulst AC, Houben GF Accidental food allergy reactions: Products and undeclared ingredients (2018) Journal of Allergy and Clinical Immunology, 142 (3), pp. 865–875. 10.1016/j.jaci.2018.04.041 - DOI - PubMed
-
- Blom WM, Remington BC, Baumert JL, Bucchini L, Crépet A, Crevel RWR, Madsen CB, Taylor SL, Houben GF, Kruizinga AG Sensitivity analysis to derive a food consumption point estimate for deterministic food allergy risk assessment (2019) Food and Chemical Toxicology, 125, pp. 413–421. 10.1016/j.fct.2019.01.025 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical