Tuning tuft cells: new ligands and effector functions reveal tissue-specific function
- PMID: 33166855
- PMCID: PMC7925335
- DOI: 10.1016/j.coi.2020.09.006
Tuning tuft cells: new ligands and effector functions reveal tissue-specific function
Abstract
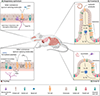
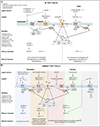
Tuft cells are rare chemosensory epithelial cells that monitor their environment and relay messages to the surrounding tissue via secretion of neuromodulatory and immunomodulatory molecules. In the small intestine tuft cells detect helminth infection, protist colonization, and bacterial dysbiosis, and initiate a type 2 immune response characterized by tissue remodeling. In the airways, tuft cells sense bacteria, allergens, and noxious stimuli and drive evasive behavior, neuroinflammation, and anti-bacterial responses. Here we summarize the most recent tuft cell research and discuss how these findings have provided insight into tuft cell diversity. Built around a core program of chemosensing, tuft cell receptors and effector functions are tuned to the unique environmental exposure and physiology of their surrounding tissue.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures
References
-
- Järvi O, Keyriläinen O: On the cellular structures of the epithelial invasions in the gladular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand 1956, 38:72–73. - PubMed
-
- Rhodin J, Dalhamn T: Electron microscopy of the tracheal ciliated mucosa in rat. Zeitschrift für Zellforsch und Mikroskopische Anat 1956, 44:345–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

