COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice
- PMID: 33166988
- PMCID: PMC7855185
- DOI: 10.1038/s41586-020-2943-z
COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice
Abstract
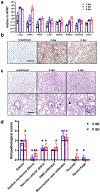
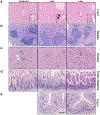
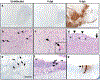
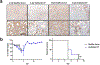
The ongoing coronavirus disease 2019 (COVID-19) pandemic is associated with substantial morbidity and mortality. Although much has been learned in the first few months of the pandemic, many features of COVID-19 pathogenesis remain to be determined. For example, anosmia is a common presentation, and many patients with anosmia show no or only minor respiratory symptoms1. Studies in animals infected experimentally with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19, provide opportunities to study aspects of the disease that are not easily investigated in human patients. Although the severity of COVID-19 ranges from asymptomatic to lethal2, most experimental infections provide insights into mild disease3. Here, using K18-hACE2 transgenic mice that were originally developed for SARS studies4, we show that infection with SARS-CoV-2 causes severe disease in the lung and, in some mice, the brain. Evidence of thrombosis and vasculitis was detected in mice with severe pneumonia. Furthermore, we show that infusion of convalescent plasma from a recovered patient with COVID-19 protected against lethal disease. Mice developed anosmia at early time points after infection. Notably, although pre-treatment with convalescent plasma prevented most signs of clinical disease, it did not prevent anosmia. Thus, K18-hACE2 mice provide a useful model for studying the pathological basis of both mild and lethal COVID-19 and for assessing therapeutic interventions.
Figures




Update of
-
K18-hACE2 Mice for Studies of COVID-19 Treatments and Pathogenesis Including Anosmia.bioRxiv [Preprint]. 2020 Aug 10:2020.08.07.242073. doi: 10.1101/2020.08.07.242073. bioRxiv. 2020. Update in: Nature. 2021 Jan;589(7843):603-607. doi: 10.1038/s41586-020-2943-z. PMID: 32817939 Free PMC article. Updated. Preprint.
References
Extended data references
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

