Using a Respectful Approach to Child-centred Healthcare (ReACH) in a paediatric clinical trial: A feasibility study
- PMID: 33166989
- PMCID: PMC7652280
- DOI: 10.1371/journal.pone.0241764
Using a Respectful Approach to Child-centred Healthcare (ReACH) in a paediatric clinical trial: A feasibility study
Abstract
Background: There is a growing momentum in paediatric ethics to develop respectful research and healthcare protocols. We developed, tested and refined our 'Respectful Approach to Child-centred Healthcare' (ReACH), to underpin respectful participant interactions in a clinical trial.
Objective: To determine whether a ReACH-based approach is acceptable to children and parents, and effective in obtaining compliance with common healthcare assessments in a clinical trial of healthy 4-6-year-old children.
Methods: ReACH-based child assessments were evaluated at two baseline clinics and one post-intervention, using mixed methods. Children (n = 49; 46.9% female; mean age = 5.24±0.88 years at baseline) and their parents provided independent evaluation, via customised 5-point Likert scales and qualitative feedback. A dedicated child researcher evaluated adherence to the study ReACH principles.
Results: Children achieved compliance rates of 95% for body composition (BodPod) assessments; 89% for blood pressure measurements, and 92% (baseline) and 87% (post-intervention) for blood draws. Adherence to ReACH principles during clinic visits was positively associated with child compliance, significantly for baseline BodPod (p = 0.002) and blood test (p = 0.009) clinics. Satisfaction with BodPod protocols was positively associated with compliance, for children at baseline (p = 0.029) and for parents post-intervention (p <0.001). Parents rated the study itself very highly, with 91.7% satisfied at baseline and 100% post-intervention. Qualitative feedback reflected an enjoyable study experience for both parents and children.
Conclusions: Adherence to our emerging ReACH approach was associated with high child compliance rates for common healthcare assessments, although no causality can be inferred at this preliminary stage of development. Participants expressed satisfaction with all aspects of the study. Our use of child-centred methods throughout a research intervention appears feasible and acceptable to children and their parents.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
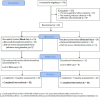



References
-
- United Nations Children's Fund [UNICEF]. United Nations Convention on the Rights of the Child Geneva: United Nations1989. p. 1–14.
-
- Declaration of Helsinki: ethical principles for medical research involving human subjects [Internet]. World Medical Association [WMA]. 2013 [cited December 10, 2019]. Available from: http://www.wma.net/en/30publications/10policies/b3/.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

