Cellular Reprogramming-A Model for Melanoma Cellular Plasticity
- PMID: 33167306
- PMCID: PMC7663830
- DOI: 10.3390/ijms21218274
Cellular Reprogramming-A Model for Melanoma Cellular Plasticity
Abstract
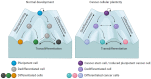
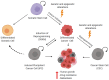
Cellular plasticity of cancer cells is often associated with phenotypic heterogeneity and drug resistance and thus remains a major challenge for the treatment of melanoma and other types of cancer. Melanoma cells have the capacity to switch their phenotype during tumor progression, from a proliferative and differentiated phenotype to a more invasive and dedifferentiated phenotype. However, the molecular mechanisms driving this phenotype switch are not yet fully understood. Considering that cellular heterogeneity within the tumor contributes to the high plasticity typically observed in melanoma, it is crucial to generate suitable models to investigate this phenomenon in detail. Here, we discuss the use of complete and partial reprogramming into induced pluripotent cancer (iPC) cells as a tool to obtain new insights into melanoma cellular plasticity. We consider this a relevant topic due to the high plasticity of melanoma cells and its association with a strong resistance to standard anticancer treatments.
Keywords: cellular plasticity; heterogeneity; melanoma; partial reprogramming; phenotype switch.
Conflict of interest statement
None of the authors have potential conflicts of interest to be disclosed. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Melanoma Progression Inhibits Pluripotency and Differentiation of Melanoma-Derived iPSCs Produces Cells with Neural-like Mixed Dysplastic Phenotype.Stem Cell Reports. 2019 Jul 9;13(1):177-192. doi: 10.1016/j.stemcr.2019.05.018. Epub 2019 Jun 20. Stem Cell Reports. 2019. PMID: 31231022 Free PMC article.
-
Directed Dedifferentiation Using Partial Reprogramming Induces Invasive Phenotype in Melanoma Cells.Stem Cells. 2016 Apr;34(4):832-46. doi: 10.1002/stem.2284. Epub 2016 Jan 19. Stem Cells. 2016. PMID: 26753613
-
Incomplete cellular reprogramming of colorectal cancer cells elicits an epithelial/mesenchymal hybrid phenotype.J Biomed Sci. 2018 Jul 19;25(1):57. doi: 10.1186/s12929-018-0461-1. J Biomed Sci. 2018. PMID: 30025541 Free PMC article.
-
Epigenetics and metabolism at the crossroads of stress-induced plasticity, stemness and therapeutic resistance in cancer.Theranostics. 2020 May 15;10(14):6261-6277. doi: 10.7150/thno.42523. eCollection 2020. Theranostics. 2020. PMID: 32483452 Free PMC article. Review.
-
How Neural Crest Transcription Factors Contribute to Melanoma Heterogeneity, Cellular Plasticity, and Treatment Resistance.Int J Mol Sci. 2021 May 28;22(11):5761. doi: 10.3390/ijms22115761. Int J Mol Sci. 2021. PMID: 34071193 Free PMC article. Review.
Cited by
-
Rho-Associated Protein Kinase Activity Is Required for Tissue Homeostasis in the Xenopus laevis Ciliated Epithelium.J Dev Biol. 2024 Jun 11;12(2):17. doi: 10.3390/jdb12020017. J Dev Biol. 2024. PMID: 38921484 Free PMC article.
-
Extracellular vesicles modulate integrin signaling and subcellular energetics to promote pulmonary lymphangioleiomyomatosis metastasis.Res Sq [Preprint]. 2025 Mar 20:rs.3.rs-5390547. doi: 10.21203/rs.3.rs-5390547/v1. Res Sq. 2025. PMID: 40166013 Free PMC article. Preprint.
-
Polymer Thin Film Promotes Tumor Spheroid Formation via JAK2-STAT3 Signaling Primed by Fibronectin-Integrin α5 and Sustained by LMO2-LDB1 Complex.Biomedicines. 2022 Oct 24;10(11):2684. doi: 10.3390/biomedicines10112684. Biomedicines. 2022. PMID: 36359204 Free PMC article.
-
NRF2 and Key Transcriptional Targets in Melanoma Redox Manipulation.Cancers (Basel). 2022 Mar 16;14(6):1531. doi: 10.3390/cancers14061531. Cancers (Basel). 2022. PMID: 35326683 Free PMC article. Review.
-
Inactivation of Tumor Suppressor CYLD Inhibits Fibroblast Reprogramming to Pluripotency.Cancers (Basel). 2023 Oct 15;15(20):4997. doi: 10.3390/cancers15204997. Cancers (Basel). 2023. PMID: 37894364 Free PMC article.
References
-
- Tsoi J., Robert L., Paraiso K., Galvan C., Sheu K.M., Lay J., Wong D.J.L., Atefi M., Shirazi R., Wang X., et al. Multi-stage Differentiation Defines Melanoma Subtypes with Differential Vulnerability to Drug-Induced Iron-Dependent Oxidative Stress. Cancer Cell. 2018;33:890–904. doi: 10.1016/j.ccell.2018.03.017. - DOI - PMC - PubMed
-
- Yuan S., Norgard R.J., Stanger B.Z. Cellular plasticity in cancer. Cancer Discov. 2019;9:837–851. doi: 10.1158/2159-8290.CD-19-0015. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

