Extracellular Vesicles and the Oviduct Function
- PMID: 33167378
- PMCID: PMC7663821
- DOI: 10.3390/ijms21218280
Extracellular Vesicles and the Oviduct Function
Abstract
In mammals, the oviduct (or the Fallopian tube in humans) can be divided into the infundibulum (responsible for oocyte pick-up), ampulla (site of fertilization), isthmus (where preimplantation embryos develop), and uterotubal junction (where embryos transit to the uterus). The oviductal fluid, as well as extracellular vesicles produced from the oviduct epithelial cells, referred to as oEVs, have been shown to improve the fertilization process, prevent polyspermy, and aid in embryo development. oEVs contain molecular cargos (such as miRNAs, mRNAs, proteins, and lipids) that can be delivered and fuse to recipient cells. oEVs produced from the ampulla appear to be functionally distinct from those produced from the isthmus. In multiple species including mice, cats, dogs, pigs, and cows, oEVs can be incorporated into the oocytes, sperm, and embryos. In this review, we show the positive impact of oEVs on gamete function as well as blastocyst development and how they may improve embryo quality in in vitro conditions in an assisted reproductive technology setting for rodents, domestic animals, farm animals, and humans.
Keywords: egg; embryo; exosome; extracellular vesicle; fallopian tube; microvesicle; oocyte; oviduct; oviductosome; sperm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
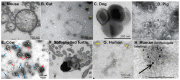
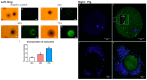
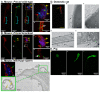

References
Publication types
MeSH terms
Grants and funding
- R01 HD097087/HD/NICHD NIH HHS/United States
- R01HD097087-02S1/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- (LSAMP)/Louis Stokes Alliance for Minority Participation Research Award
- R01HD097087/Eunice Kennedy Shriver National Institute of Child Health and Human Development
LinkOut - more resources
Full Text Sources
Miscellaneous

