One-Month Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention With Zotarolimus-Eluting Stents in High-Bleeding-Risk Patients
- PMID: 33167705
- PMCID: PMC7665241
- DOI: 10.1161/CIRCINTERVENTIONS.120.009565
One-Month Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention With Zotarolimus-Eluting Stents in High-Bleeding-Risk Patients
Abstract
Background: Despite treatment guidance endorsing shortened dual antiplatelet therapy (DAPT) duration in high bleeding risk (HBR) patients after drug-eluting stents, limited evidence exists to support these recommendations. The present study was designed to examine the safety and effectiveness of 1-month DAPT duration following percutaneous coronary intervention with zotarolimus-eluting stents in HBR patients.
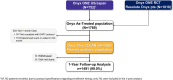
Methods: Onyx ONE Clear was a prospective, multicenter, nonrandomized study evaluating the safety and effectiveness of 1-month DAPT followed by single antiplatelet therapy in HBR patients undergoing percutaneous coronary intervention with Resolute Onyx drug-eluting stents. The primary analysis of cardiac death or myocardial infarction between 1 month and 1 year was performed in the prespecified one-month clear population of patients pooled from the Onyx ONE US/Japan study and Onyx ONE randomized controlled trial. One-month clear was defined as DAPT adherence and without major adverse events during the first month following percutaneous coronary intervention.
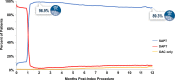
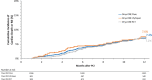
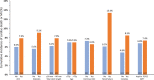
Results: Among patients enrolled in Onyx ONE US/Japan (n=752) and Onyx ONE randomized controlled trial (n=1018), 1506 patients fulfilled one-month clear criteria. Mean HBR characteristics per patient was 1.6 with 44.7% having multiple risks. By 2 months and 1 year, respectively, 96.9% and 89.3% of patients were taking single antiplatelet therapy. Between 1 month and 1 year, the rate of the primary end point was 7.0%. The 1-sided upper 97.5% CI was 8.4%, less than the performance goal of 9.7% (P<0.001).
Conclusions: Among HBR patients who were event free before DAPT discontinuation at 1 month, favorable safety and effectiveness through 1 year support treatment with Resolute Onyx drug-eluting stents as part of an individualized strategy for shortened DAPT duration following percutaneous coronary intervention. Registration: URL: https://www.clinicaltrials.gov; Unique identifier NCT03647475.
Keywords: Japan; drug-eluting stents; goals; hemorrhage; stents.
Conflict of interest statement
Dr Kandzari reports institutional research/grant support from Biotronik, Boston Scientific, Medinol, Medtronic, OrbusNeich, and Teleflex and personal consulting honoraria from Biotronik, Cardiovascular Systems, Inc, and Medtronic. Dr Kirtane reports institutional funding to Columbia University or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, Cardiovascular Systems, Inc, Philips, and ReCor Medical; personal: Continuing Medical Education program honoraria and travel/meal reimbursements only. Dr Windecker reports research and educational grants to the institution from Abbott, Amgen, Bristol-Myers Squibb, Bayer, Boston Scientific, Biotronik, Cardinal Health, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Johnson & Johnson, Medtronic, Querbet, Polares, Sanofi, Terumo, and Sinomed. Dr Latib reports institutional research/grant support from Abbott, Boston Scientific, and Medtronic and personal consulting honoraria from Abbott and Medtronic. Dr Kedhi reports speaker honoraria from Medtronic, Abbott, Meril, and Daiichi Sankyo. Dr Mehran reports institutional research grants from Abbott Laboratories, AstraZeneca, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, Cardiovascular European Research Center, Chiesi, Concept Medical, CSL Behring, Daiichi-Sankyo, Inc, Medtronic, Novartis Pharmaceuticals, and OrbusNeich; consultant fees from Abbott Laboratories, Boston Scientific, Janssen Scientific Affairs, Medscape/WebMD, Medtelligence (Janssen Scientific Affairs), Roivant Sciences, Sanofi, and Siemens Medical Solutions; consultant fees paid to the institution from Abbott Laboratories and Bristol-Myers Squibb; advisory board, funding paid to the institution from Spectranetics/Philips/Volcano Corp; is a consultant (spouse) to Abiomed, The Medicines Company; equity <1% from Claret Medical and Elixir Medical; Data and Safety Monitoring Board Membership fees paid to the institution from Watermark Research Partners; consulting (no fee) from Idorsia Pharmaceuticals, Ltd and Regeneron Pharmaceuticals; is an associate editor for American College of Cardiology and American Medical Association. Dr Price reports institutional research/grant support from Daiichi Sankyo and personal consulting or speaking honoraria from Abbott Vascular, AstraZeneca, ACIST Medical, Boston Scientific, Chiesi USA, Medtronic, and W.L. Gore Medical. Dr Simon reports institutional research/grant support from Medtronic and personal educational honoraria from Medtronic. Dr Worthley reports research grants for Abbott and Biotronik. Dr Zaman reports consulting fees from Abbott, Boston Scientific, Medtronic, Meril, Sahajanand Medical Technologies, and Terumo. Dr Choi reports consulting fees from Medtronic. Dr Caputo reports consulting and speaking honoraria from Medtronic, Co, Merit Medical, Edwards Lifesciences, and Cardinal Health. Dr Potluri reports grant support, speaker or proctor, and honoraria from Terumo, Edwards, Medtronic, Boston Scientific, Cordis, Abbott, Philips, and Janssen. Dr Nazif reports institutional research/grant support from Medtronic and Boston Scientific and personal consulting fees or honoraria from Medtronic and Boston Scientific. M. Parke, Dr Lee, and Dr Lung are employees and shareholders of Medtronic. Dr Stone reports speaker or other honoraria from Cook, Terumo, QOOL Therapeutics, and Orchestra Biomed; is a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme; and reports equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. The other authors report no conflicts.
Figures
References
-
- Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et al. 2016 ACC/AHA Guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134:e123–e155. doi: 10.1161/CIR.0000000000000404 - PubMed
-
- Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Jüni P, Kastrati A, Kolh P, Mauri L, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419 - PubMed
-
- Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, et al. ; ZEUS Investigators. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805–815. doi: 10.1016/j.jacc.2014.11.053 - PubMed
-
- Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, et al. ; SENIOR Investigators. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391:41–50. doi: 10.1016/S0140-6736(17)32713-7 - PubMed
-
- Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, et al. ; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. doi: 10.1056/NEJMoa1503943 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

