DeepCryoPicker: fully automated deep neural network for single protein particle picking in cryo-EM
- PMID: 33167860
- PMCID: PMC7653784
- DOI: 10.1186/s12859-020-03809-7
DeepCryoPicker: fully automated deep neural network for single protein particle picking in cryo-EM
Abstract
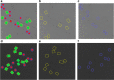
Background: Cryo-electron microscopy (Cryo-EM) is widely used in the determination of the three-dimensional (3D) structures of macromolecules. Particle picking from 2D micrographs remains a challenging early step in the Cryo-EM pipeline due to the diversity of particle shapes and the extremely low signal-to-noise ratio of micrographs. Because of these issues, significant human intervention is often required to generate a high-quality set of particles for input to the downstream structure determination steps.
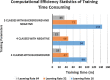
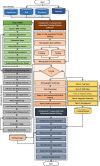
Results: Here we propose a fully automated approach (DeepCryoPicker) for single particle picking based on deep learning. It first uses automated unsupervised learning to generate particle training datasets. Then it trains a deep neural network to classify particles automatically. Results indicate that the DeepCryoPicker compares favorably with semi-automated methods such as DeepEM, DeepPicker, and RELION, with the significant advantage of not requiring human intervention.
Conclusions: Our framework combing supervised deep learning classification with automated un-supervised clustering for generating training data provides an effective approach to pick particles in cryo-EM images automatically and accurately.
Keywords: AutoCryoPicker; Cryo-EM; Deep learning; Intensity based clustering (IBC); Micrograph; Protein structure determination; Singe particle pickling; Super clustering; SuperCryoPicker.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures



















References
-
- Frank J. Three-dimensional electron microscopy of macromolecular assemblies. New York: Oxford University Press; 2006.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

