DNA methylation in infants with low and high body fatness
- PMID: 33167873
- PMCID: PMC7654595
- DOI: 10.1186/s12864-020-07169-7
DNA methylation in infants with low and high body fatness
Abstract
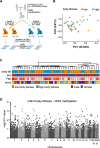
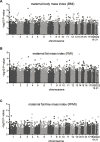
Background: Birth weight is determined by the interplay between infant genetics and the intrauterine environment and is associated with several health outcomes in later life. Many studies have reported an association between birth weight and DNA methylation in infants and suggest that altered epigenetics may underlie birthweight-associated health outcomes. However, birth weight is a relatively nonspecific measure of fetal growth and consists of fat mass and fat-free mass which may have different effects on health outcomes which motivates studies of infant body composition and DNA methylation. Here, we combined genome-wide DNA methylation profiling of buccal cells from 47 full-term one-week old infants with accurate measurements of infant fat mass and fat-free mass using air-displacement plethysmography.
Results: No significant association was found between DNA methylation in infant buccal cells and infant body composition. Moreover, no association between infant DNA methylation and parental body composition or indicators of maternal glucose metabolism were found.
Conclusions: Despite accurate measures of body composition, we did not identify any associations between infant body fatness and DNA methylation. These results are consistent with recent studies that generally have identified only weak associations between DNA methylation and birthweight. Although our results should be confirmed by additional larger studies, our findings may suggest that differences in DNA methylation between individuals with low and high body fatness may be established later in childhood.
Conflict of interest statement
Authors declare no competing interests.
Figures
References
-
- Soubry A, Schildkraut JM, Murtha A, Wang F, Huang Z, Bernal A, Kurtzberg J, Jirtle RL, Murphy SK, Hoyo C. Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a newborn epigenetics study (NEST) cohort. BMC Med. 2013;11:29. doi: 10.1186/1741-7015-11-29. - DOI - PMC - PubMed

