Efficacy and efficiency of a new therapeutic approach based on activity-oriented proprioceptive antiedema therapy (TAPA) for edema reduction and improved occupational performance in the rehabilitation of breast cancer-related arm lymphedema in women: a controlled, randomized clinical trial
- PMID: 33167921
- PMCID: PMC7652582
- DOI: 10.1186/s12885-020-07558-x
Efficacy and efficiency of a new therapeutic approach based on activity-oriented proprioceptive antiedema therapy (TAPA) for edema reduction and improved occupational performance in the rehabilitation of breast cancer-related arm lymphedema in women: a controlled, randomized clinical trial
Abstract
Background: Breast cancer (BC) is a major public health issue. More than one out of five women treated for breast cancer will develop lymphedema in an upper extremity. Current evidence advocates transdisciplinary oncological rehabilitation. Therefore, research in this area is necessary since limited consensus having been reached with regard to the basic essential components of this rehabilitation. Consensus has, however, been reached on the use of decongestive lymphedema therapy (DLT), but due to a lack of tests, the necessary dosages are unknown and its level is moderately strong. This study attempts to verify both the efficacy of activity-oriented proprioceptive antiedema therapy (TAPA), as compared to conventional treatments such as DLT or Complex Physical Therapy (CPT), as well as its efficiency in terms of cost-effectiveness, for patients affected by breast cancer-related arm lymphedema.
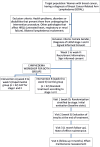
Methods: Controlled, randomized clinical trial with dual stratification, two parallel arms, longitudinal and single blind. 64 women with breast cancer-related arm lymphedema will take part in the study. The experimental group intervention will be the same for stage I and II, and will consist of neuro-dynamic exercises oriented to the activity, proprioceptive neuromuscular facilitation activities and proprioceptive anti-edema bandaging. The control group intervention, depending on the stage, will consist of preventive measures, skin care and exercise-prescribed training in the lymphedema workshop as well as compression garments (Stage I) or conservative Complex Decongestive Therapy treatment (skin care, multi-layer bandaging, manual lymphatic drainage and massage therapy) (Stage II).
Results: Sociodemographic and clinical variables will be collected for the measurement of edema volume and ADL performance. Statistical analysis will be performed on intent to treat.
Discussion: It has been recommended that patient training be added to DLT, as well as a re-designing of patient lifestyles and the promotion of health-related aspects. In addition, clinical trials should be undertaken to assess neural mobilization techniques and proprioceptive neuromuscular facilitation should be included in the therapy. Cohesive bandaging will also be performed as an early form of pressotherapy. The proposed study combines all of these aspects in order to increased comfort and promote the participation of individuals with lymphedema in everyday situations.
Limitations: The authors have proposed the assessment of the experimental treatment for stages I and II. One possible limitation is the lack of awareness of whether or not this treatment would be effective for other stages as well as the concern for proper hand cleansing during use of bandages, given the current COVID-19 pandemic situation.
Trial registration: This trial was registered in ClinicalTrials.gov ( NCT03762044 ). Date of registration: 23 November 2018. Prospectively Registered.
Keywords: Activity-oriented proprioceptive antiedema therapy (TAPA in Spanish); Breast cancer (BC) - lymphedema (L); Complex decongestive therapy (CDT); Health-related quality of life (HRQOL); Occupational therapy (OT).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial.J Clin Med. 2022 Apr 16;11(8):2234. doi: 10.3390/jcm11082234. J Clin Med. 2022. PMID: 35456327 Free PMC article.
-
Effect of physical therapy on breast cancer related lymphedema: protocol for a multicenter, randomized, single-blind, equivalence trial.BMC Cancer. 2014 Apr 3;14:239. doi: 10.1186/1471-2407-14-239. BMC Cancer. 2014. PMID: 24708851 Free PMC article. Clinical Trial.
-
Complex Decongestive Therapy Enhances Upper Limb Functions in Patients with Breast Cancer-Related Lymphedema.Lymphat Res Biol. 2018 Oct;16(5):446-452. doi: 10.1089/lrb.2017.0061. Epub 2018 Jan 22. Lymphat Res Biol. 2018. PMID: 29356592
-
Effectiveness of complete decongestive therapy for upper extremity breast cancer-related lymphedema: a review of systematic reviews.Med Oncol. 2024 Oct 23;41(11):297. doi: 10.1007/s12032-024-02421-6. Med Oncol. 2024. PMID: 39438358 Free PMC article.
-
Complete Decongestive Therapy Effect on Breast Cancer Related to Lymphedema: A Systemic Review and Meta-Analysis of Randomized Controlled Trials.Asian Pac J Cancer Prev. 2023 Jul 1;24(7):2225-2238. doi: 10.31557/APJCP.2023.24.7.2225. Asian Pac J Cancer Prev. 2023. PMID: 37505751 Free PMC article.
Cited by
-
Activity-Oriented Antiedema Proprioceptive Therapy (TAPA) for Shoulder Mobility Improvement in Women with Upper Limb Lymphedema Secondary to Breast Cancer: A Multicenter Controlled Clinical Trial.J Clin Med. 2022 Apr 16;11(8):2234. doi: 10.3390/jcm11082234. J Clin Med. 2022. PMID: 35456327 Free PMC article.
-
Accuracy, Sensitivity, and Specificity of the LLIS and ULL27 in Detecting Breast Cancer-Related Lymphedema.Ann Surg Oncol. 2022 Jan;29(1):438-445. doi: 10.1245/s10434-021-10469-1. Epub 2021 Jul 15. Ann Surg Oncol. 2022. PMID: 34264409 Free PMC article.
-
Effect of Psychological Intervention-Assisted Comfort Nursing Based on PERMA Model on Stress and Psychological Changes of Patients after Breast Cancer Surgery.Comput Math Methods Med. 2022 Jun 7;2022:1853754. doi: 10.1155/2022/1853754. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Sep 27;2023:9836745. doi: 10.1155/2023/9836745. PMID: 35712008 Free PMC article. Retracted.
-
Acute Effects of Remedial Exercises with and without Compression on Breast-Cancer-Related Lymphedema.Healthcare (Basel). 2023 Nov 11;11(22):2949. doi: 10.3390/healthcare11222949. Healthcare (Basel). 2023. PMID: 37998441 Free PMC article.
-
Emerging Anti-Inflammatory Pharmacotherapy and Cell-Based Therapy for Lymphedema.Int J Mol Sci. 2022 Jul 9;23(14):7614. doi: 10.3390/ijms23147614. Int J Mol Sci. 2022. PMID: 35886961 Free PMC article. Review.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Asociación Española Contra el Cáncer (Spanish Association Againts Cancer). Todo lo que Necesitas Saber (All you need to know). Available from: https://www.aecc.es/es/todo-sobre-cancer/tipos-cancer/cancer-mama. Accessed 13 Mar 2019.
-
- Baxter MF, Newman R, Longpré SM, Polo KM. Occupational therapy’s role in cancer survivorship as a chronic condition. Am J Occup Ther. 2017;71(3):7103090010P1–P7. - PubMed
-
- Cuello Villaverde E, Guerola Soler N, López RA. Perfil clínico y terapéutico del linfedema postmastectomía. Rehabilitación. 2003;37(1):22–32. doi: 10.1016/S0048-7120(03)73329-5. - DOI
-
- Puigdellivol Serafí C, Alonso Álvarez B. Guia de orientación diagnóstica y terapéutica del linfedema [Internet]. 2017. 148 p. Available from: http://www.capitulodeflebologia.org/media/Guia-linfedema-segunda-edicion....
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials