Scleroderma-specific autoantibodies embedded in immune complexes mediate endothelial damage: an early event in the pathogenesis of systemic sclerosis
- PMID: 33168071
- PMCID: PMC7654597
- DOI: 10.1186/s13075-020-02360-3
Scleroderma-specific autoantibodies embedded in immune complexes mediate endothelial damage: an early event in the pathogenesis of systemic sclerosis
Abstract
Background: Consistently with their diagnostic and prognostic value, autoantibodies specific for systemic sclerosis (SSc) embedded in immune complexes (ICs) elicited a pro-inflammatory and pro-fibrotic cascade in healthy skin fibroblasts, engaging Toll-like receptors (TLRs) via their nucleic acid components. The objective of this study was to investigate the pathogenicity of SSc-ICs in endothelial cells.
Methods: ICs were purified from the sera of SSc patients bearing different autoantibody specificities (antibodies against DNA topoisomerase I, centromeric proteins, RNA polymerase, and Th/To), patients with systemic lupus erythematosus (SLE) and primary anti-phospholipid syndrome (PAPS), or healthy controls (NHS) using polyethylene glycol precipitation. Human umbilical vein endothelial cells (HUVECs) were incubated with ICs, positive and negative controls. mRNA levels of endothelin-1 (et-1), collagenIα1 (colIα1), interferon (IFN)-α, and IFN-β were investigated by real-time PCR; et-1 and il-6 mRNA levels were assessed after pre-treatment with bafilomycin. ICAM-1 expression was evaluated by cell ELISA; secretion of IL-6, IL-8, and transforming growth factor (TGF)-β1 in culture supernatants was measured by ELISA. The expression of Fcγ receptors (CD64, CD32, and CD16) was assessed in endothelial cells at FACS analysis. Intracellular signaling pathways culminating with NFκB, p38MAPK, SAPK-JNK, and Akt were assessed by Western blotting. Healthy skin fibroblasts were stimulated with supernatants from HUVECs incubated with ICs, and TGF-β1 secretion and mRNA levels of colIα1 and matrix metalloproteinase (mmp)-1, protein expression of α smooth muscle actin (α-SMA), and IL-6 were evaluated by Western blotting; et-1 mRNA levels were assessed in fibroblasts pre-treated with IL-6 and TGF-β inhibitors and stimulated with ATA-ICs.
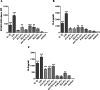
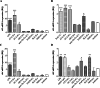
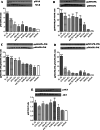
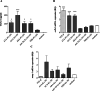
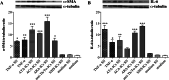
Results: All SSc stimulated IL-6 secretion; ACA-ICs and anti-Th/To-ICs increased ICAM-1 expression; all SSc-ICs but anti-Th/To-ICs augmented IL-8 levels; all SSc-ICs but ACA-ICs and ARA-ICs upregulated et-1, and all SSc-ICs but ARA-ICs affected TGF-β1 secretion. colIα1, IFN-α, and IFN-β mRNA levels were not affected by any SSc-IC. FcγRII (CD32) and FcγRIII (CD16) were not detectable on HUVECs, while FcγRI (CD64) was minimally expressed. A differential modulation of tlr expression was observed: tlr2, tlr3, and tlr4 were upregulated by ATA-ICs and ACA-ICs, while anti-Th/To-ICs resulted in tlr9 upregulation. Pre-treatment with bafilomycin did not affect the upregulation of et-1 and il-6 induced by ATA-ICs, ACA-ICs, and anti-Th/To-ICs; a 23% reduction in both genes was reported for ARA-ICs. All SSc-ICs activated p38MAPK and Akt, and all SSc-ICs but ARA-ICs yielded the activation of NFκB; ATA-ICs and ACA-ICs increased the activation rate of both subunits of SAPK-JNK. When healthy skin fibroblasts were stimulated with supernatants from HUVECs incubated with SSc-ICs, TGF-β1 secretion, colIα1, α-SMA, and IL-6 expression levels were significantly modulated. Pre-treatment with IL-6 and TGF-β inhibitors prevented et-1 upregulation induced by ATA-ICs by 85% and 77%, respectively.
Conclusions: These data provide the first demonstration of the pathogenicity of ICs from scleroderma patients with different autoantibodies on the endothelium. Endothelial activation induced by SSc-ICs ultimately led to a pro-fibrotic phenotype in healthy skin fibroblasts.
Keywords: Autoantibodies; Endothelial cells; Fibrosis; Immune complexes; Inflammation; Systemic sclerosis; Toll-like receptors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

