Negative pressure wound therapy (NPWT) on closed incisions to prevent surgical site infection in high-risk patients in hepatopancreatobiliary surgery: study protocol for a randomized controlled trial-the NP-SSI trial
- PMID: 33168081
- PMCID: PMC7654160
- DOI: 10.1186/s13063-020-04831-z
Negative pressure wound therapy (NPWT) on closed incisions to prevent surgical site infection in high-risk patients in hepatopancreatobiliary surgery: study protocol for a randomized controlled trial-the NP-SSI trial
Abstract
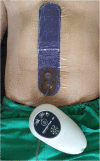
Background: Incisional surgical site infections (iSSI) in hepatopancreatobiliary (HPB) surgery usually lead to prolonged hospital stays, consume valuable resources, and impact on patients' outcome. Prophylactic closed incision negative pressure wound therapy (ciNPWT) to decrease wound complications has become available. Owing to an increasing number of studies, evidence for superiority in many indication areas has accumulated; however, in general surgery, there are a few data and those have shown contradictory results.
Methods: In this monocentric, prospective, randomized, controlled, two-armed study, the influence of ciNPWT on incisional surgical site infection rates after HPB operations will be investigated. A total of 222 patients will be randomized 1:1 to an interventional group (7-day treatment with ciNPWT) or a control group (treated with gauze dressing). The primary parameter to evaluate efficacy is the rate of incisional SSIs within 30 days after surgery. Additionally, several clinically relevant secondary outcomes will be assessed.
Discussion: A reduction in the rate of incisional SSIs would not only lead to a significant cost reduction and shorter postoperative length of stay, but may also improve postoperative quality of life for patients. While earlier publications have shown advantages for ciNPWT, recent studies did not confirm a positive effect regarding iSSI rate. Even if iSSI rate is not reduced, findings obtained from the secondary endpoints may be of clinical relevance, such as reduction of wound complication rates.
Trial registration: This trial has been registered in the German Clinical Trials Register, DRKS 00015136 . Registered on 19 February 2019 and has been approved by the local ethics committee of the University of Regensburg: 18-1225-101.
Keywords: HPB surgery; Incision management; NP-SSI; NPWT; Prevena; Randomized controlled trial; SSI; Wound infection; ciNPWT.
Conflict of interest statement
In recent years, author Frank Brennfleck has given several lectures on behalf of KCI at training events for medical professionals and has twice acted as a consultant for the company. In total, he received about 7500 Euro from the company from 2016 to 2020. The remaining authors have no conflicts of interest.
Figures

Similar articles
-
Application of Closed Incision Negative Pressure Wound Therapy in Ventral Hernia Repair Surgery Using a Polypropylene Mesh: A Randomized Clinical Trial.Medicina (Kaunas). 2024 Sep 22;60(9):1548. doi: 10.3390/medicina60091548. Medicina (Kaunas). 2024. PMID: 39336589 Free PMC article. Clinical Trial.
-
The benefit of negative pressure dressings in vascular surgery patients with infrainguinal incisions.J Vasc Surg. 2021 Nov;74(5):1668-1672. doi: 10.1016/j.jvs.2021.04.058. Epub 2021 May 18. J Vasc Surg. 2021. PMID: 34019988
-
Negative pressure wound therapy for high-risk wounds in lower extremity revascularization: study protocol for a randomized controlled trial.Trials. 2015 Nov 4;16:504. doi: 10.1186/s13063-015-1026-1. Trials. 2015. PMID: 26537879 Free PMC article. Clinical Trial.
-
Editor's Choice - Systematic Review and Meta-Analysis of Wound Adjuncts for the Prevention of Groin Wound Surgical Site Infection in Arterial Surgery.Eur J Vasc Endovasc Surg. 2021 Apr;61(4):636-646. doi: 10.1016/j.ejvs.2020.11.053. Epub 2021 Jan 7. Eur J Vasc Endovasc Surg. 2021. PMID: 33423912
-
Controlling the controls: what is negative pressure wound therapy compared to in clinical trials?Colorectal Dis. 2023 Apr;25(4):794-805. doi: 10.1111/codi.16465. Epub 2023 Jan 23. Colorectal Dis. 2023. PMID: 36579358 Review.
Cited by
-
A Systematic Review of Closed-Incision Negative-Pressure Wound Therapy for Hepato-Pancreato-Biliary Surgery: Updated Evidence, Context, and Clinical Implications.J Clin Med. 2025 Jul 22;14(15):5191. doi: 10.3390/jcm14155191. J Clin Med. 2025. PMID: 40806812 Free PMC article. Review.
-
Application of Closed Incision Negative Pressure Wound Therapy in Ventral Hernia Repair Surgery Using a Polypropylene Mesh: A Randomized Clinical Trial.Medicina (Kaunas). 2024 Sep 22;60(9):1548. doi: 10.3390/medicina60091548. Medicina (Kaunas). 2024. PMID: 39336589 Free PMC article. Clinical Trial.
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2022 Apr 26;4(4):CD009261. doi: 10.1002/14651858.CD009261.pub7. Cochrane Database Syst Rev. 2022. PMID: 35471497 Free PMC article.
References
-
- Centers for Disease Control and Prevention. National and State Healthcare Associated Infections Progress Report 2016.
-
- European Centre for Disease Prevention and Control. Surveillance of surgical site infections in Europe: 2010–2011.
-
- European Centre for Disease Prevention and Control. Point prevalence survey of healthcare - associated infections and antimicrobial use in European acute care hospitals: 2011–2012.. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

