Epidemiology of rotavirus diarrhea among children less than 5 years hospitalized with acute gastroenteritis prior to rotavirus vaccine introduction in India
- PMID: 33168345
- PMCID: PMC7694878
- DOI: 10.1016/j.vaccine.2020.10.084
Epidemiology of rotavirus diarrhea among children less than 5 years hospitalized with acute gastroenteritis prior to rotavirus vaccine introduction in India
Abstract
Background: Rotavirus is an important cause of severe diarrhea requiring hospitalization, accounting for approximately 78,000 deaths annually in Indian children below 5 years of age. We present epidemiological data on severe rotavirus disease collected during hospital-based surveillance in India before the introduction of the oral rotavirus vaccine into the national immunization schedule.
Methods: The National Rotavirus Surveillance Network was created involving 28 hospital sites and 11 laboratories across the four geographical regions of India. From September 2012 to August 2016 children less than 5 years of age hospitalized for diarrhea for at least 6 h, were enrolled. After recording clinical details, a stool sample was collected from each enrolled child, which was tested for rotavirus antigen using enzyme immunoassay (EIA). Nearly 2/3rd of EIA positive samples were genotyped using reverse transcription polymerase chain reaction to identify the G and P types.
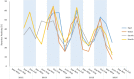
Results: Of the 21,421 children enrolled during the 4 years surveillance, 36.3% were positive for rotavirus. The eastern region had the highest proportion of rotavirus associated diarrhea (39.8%), while the southern region had the lowest (33.8%). Rotavirus detection rates were the highest in children aged 6-23 months (41.8%), and 24.7% in children aged < 6 months. Although rotavirus associated diarrhea was seen throughout the year, the highest positivity was documented between December and February across all the regions. The most common rotavirus genotype was G1P[8] (52.9%), followed by G9P4 (8.7%) and G2P4 (8.4%).
Conclusions: There is high burden of rotavirus gastroenteritis among Indian children below 5 years of age hospitalized for acute diarrhea thereby highlighting the need for introduction of rotavirus vaccine into the national immunization program and also for monitoring circulating genotypes.
Keywords: Children; Diarrhea; India; Rotavirus; Surveillance.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- WHO. Rotavirus. https://www.who.int/ith/diseases/rotavirus/en/ (Accessed 12 August 2019).
-
- WHO. Rotavirus. https://www.who.int/immunization/diseases/rotavirus/en/ (Accessed 12 August 2019).
-
- John J., Sarkar R., Muliyil J., Bhandari N., Bhan M.K., Kang G. Rotavirus gastroenteritis in India, 2011–2013: Revised estimates of disease burden and potential impact of vaccines. Vaccine. 2014;32(1):A5–A9. - PubMed
-
- Riddle M.S., Chen W.H., Kirkwood C.D., MacLennan C.A. Update on vaccines for enteric pathogens. Clin Microbiol Infect. 2018;24:1039–1045. - PubMed
-
- Kang G., Arora R., Chitambar S.D., Deshpande J., Gupte M.D., Kulkarni M. Multicenter, hospital-based surveillance of rotavirus disease and strains among Indian children aged <5 years. J Infect Dis. 2009;200(Suppl 1):S147–S153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

