Efficacy and safety of abatacept in active primary Sjögren's syndrome: results of a phase III, randomised, placebo-controlled trial
- PMID: 33168545
- PMCID: PMC7892395
- DOI: 10.1136/annrheumdis-2020-218599
Efficacy and safety of abatacept in active primary Sjögren's syndrome: results of a phase III, randomised, placebo-controlled trial
Abstract
Objectives: To evaluate efficacy and safety of abatacept in adults with active primary Sjögren's syndrome (pSS) in a phase III, randomised, double-blind, placebo-controlled trial.
Methods: Eligible patients (moderate-to-severe pSS [2016 ACR/European League Against Rheumatism (EULAR) criteria], EULAR Sjögren's Syndrome Disease Activity Index [ESSDAI] ≥5, anti-SS-related antigen A/anti-Ro antibody positive) received weekly subcutaneous abatacept 125 mg or placebo for 169 days followed by an open-label extension to day 365. Primary endpoint was mean change from baseline in ESSDAI at day 169. Key secondary endpoints were mean change from baseline in EULAR Sjögren's Syndrome Patient Reported Index (ESSPRI) and stimulated whole salivary flow (SWSF) at day 169. Other secondary clinical endpoints included glandular functions and patient-reported outcomes. Selected biomarkers and immune cell phenotypes were examined. Safety was monitored.
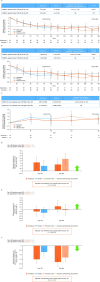
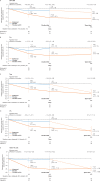
Results: Of 187 patients randomised, 168 completed double-blind period and 165 continued into open-label period. Mean (SD) baseline ESSDAI and ESSPRI total scores were 9.4 (4.3) and 6.5 (2.0), respectively. Statistical significance was not reached for primary (ESSDAI -3.2 abatacept vs -3.7 placebo, p=0.442) or key secondary endpoints (ESSPRI, p=0.337; SWSF, p=0.584). No clinical benefit of abatacept over placebo at day 169 was seen with other clinical and PRO endpoints. Relative to baseline, abatacept was associated with significant differences vs placebo in some disease-relevant biomarkers (including IgG, IgA, IgM-rheumatoid factor) and pathogenic cell subpopulations (post hoc analyses). No new safety signals were identified.
Conclusions: Abatacept treatment did not result in significant clinical efficacy compared with placebo in patients with moderate-to-severe pSS, despite evidence of biological activity.
Keywords: Sjogren's syndrome; autoimmune diseases; therapeutics.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ANB: Consultant: Bristol Myers Squibb Company, Sanofi, VielaBio; Fees: UpToDate; Clinical trials: VielaBio, Novartis. J-EG: Grant/research support: Bristol Myers Squibb Company; Consultant: Bristol Myers Squibb Company, Lilly, UCB, Sanofi-Genzyme, Pfizer. EWSC: Consulting fees and grant/research support: Bristol Myers Squibb Company; Consulting fees: AbbVie, VielaBio. TS: Grant/research support and Speakers’ bureau: Bristol Myers Squibb Company. TT: Grant/Research: AbbVie, Asahi Kasei, Astellas, AYUMI, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nipponkayaku, Novartis, Pfizer Japan, Takeda; Consultant: AbbVie, Astellas, Astra Zeneca, Chugai, Eli Lilly Japan, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Nipponkayaku, Novartis, Taiho, Taisho Toyama, UCB Japan; Speakers’ bureau: AbbVie, Astellas, Bristol Myers Squibb Company, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Novartis, Pfizer Japan, Sanofi, Takeda, Teijin. RS: Grant/research support: Pfizer; Consultant: Amgen, Bristol Myers Squibb Company, Celgene, GlaxoSmithKline, Lilly, Pfizer, Roche. GF: Consultant: Aldeyra, Allysta, Aurinia, Bristol Myers Squibb Company, Clemencia, Hovione, Kala, Lexitas PharmaServices, Nicox, Noveome, Sight Sciences, Tarsus, Tear Solutions; Stock: TearLab. MN: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. SM: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. RW: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. NR: Employee: Bristol Myers Squibb Company; Shareholder: Bristol Myers Squibb Company. HB: Unrestricted grant: Bristol Myers Squibb Company, Roche; Consultant: Speakers bureau: Bristol Myers Squibb Company, Novartis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

