Comparison of the Efficacy and Safety of Tacrolimus and Low-Dose Corticosteroid with High-Dose Corticosteroid for Minimal Change Nephrotic Syndrome in Adults
- PMID: 33168602
- PMCID: PMC7894664
- DOI: 10.1681/ASN.2019050546
Comparison of the Efficacy and Safety of Tacrolimus and Low-Dose Corticosteroid with High-Dose Corticosteroid for Minimal Change Nephrotic Syndrome in Adults
Abstract
Background: Tacrolimus is used as a steroid-sparing immunosuppressant in adults with minimal change nephrotic syndrome. However, combined treatment with tacrolimus and low-dose steroid has not been compared with high-dose steroid for induction of clinical remission in a large-scale randomized study.
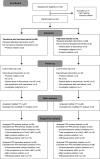
Methods: In this 24-week open-label noninferiority study, we randomized 144 adults with minimal change nephrotic syndrome to receive 0.05 mg/kg twice-daily tacrolimus plus once-daily 0.5 mg/kg prednisolone, or once-daily 1 mg/kg prednisolone alone, for up to 8 weeks or until achieving complete remission. Two weeks after complete remission, we tapered the steroid to a maintenance dose of 5-7.5 mg/d in both groups until 24 weeks after study drug initiation. The primary end point was complete remission within 8 weeks (urine protein: creatinine ratio <0.2 g/g). Secondary end points included time until remission and relapse rates (proteinuria and urine protein: creatinine ratio >3.0 g/g) after complete remission to within 24 weeks of study drug initiation.
Results: Complete remission within 8 weeks occurred in 53 of 67 patients (79.1%) receiving tacrolimus and low-dose steroid and 53 of 69 patients (76.8%) receiving high-dose steroid; this difference demonstrated noninferiority, with an upper confidence limit below the predefined threshold (20%) in both intent-to-treat (11.6%) and per-protocol (17.0%) analyses. Groups did not significantly differ in time until remission. Significantly fewer patients relapsed on maintenance tacrolimus (3-8 ng/ml) plus tapered steroid versus tapered steroid alone (5.7% versus 22.6%, respectively; P=0.01). There were no clinically relevant safety differences.
Conclusions: Combined tacrolimus and low-dose steroid was noninferior to high-dose steroid for complete remission induction in adults with minimal change nephrotic syndrome. Relapse rates were significantly lower with maintenance tacrolimus and steroid compared with steroid alone. No clinically-relevant differences in safety findings were observed.
Keywords: corticosteroid; glomerulonephritis; immunosuppression; nephrotic syndrome; remission; tacrolimus.
Copyright © 2021 by the American Society of Nephrology.
Figures




References
-
- Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al. .: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 - PubMed
-
- Szeto CC, Lai FM, Chow KM, Kwan BC, Kwong VW, Leung CB, et al. .: Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis 65: 710–718, 2015 - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2012-GN-Guideline-Eng.... Accessed October 22, 2020
-
- Deegens JK, Dijkman HB, Borm GF, Steenbergen EJ, van den Berg JG, Weening JJ, et al. .: Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 74: 1568–1576, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

