Counteracting CAR T cell dysfunction
- PMID: 33168929
- PMCID: PMC7808935
- DOI: 10.1038/s41388-020-01501-x
Counteracting CAR T cell dysfunction
Abstract
In spite of high rates of complete remission following chimeric antigen receptor (CAR) T cell therapy, the efficacy of this approach is limited by generation of dysfunctional CAR T cells in vivo, conceivably induced by immunosuppressive tumor microenvironment (TME) and excessive antigen exposure. Exhaustion and senescence are two critical dysfunctional states that impose a pivotal hurdle for successful CAR T cell therapies. Recently, modified CAR T cells with an "exhaustion-resistant" phenotype have shown superior antitumor functions and prolonged lifespan. In addition, several studies have indicated the feasibility of senescence delay in CAR T cells. Here, we review the latest reports regarding blockade of CAR T cell exhaustion and senescence with a particular focus on the exhaustion-inducing pathways. Subsequently, we describe what potential these latest insights offer for boosting the potency of adoptive cell transfer (ACT) therapies involving CAR T cells. Furthermore, we discuss how induction of costimulation, cytokine exposure, and TME modulation can impact on CAR T cell efficacy and persistence, while potential safety issues associated with reinvigorated CAR T cells will also be addressed.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
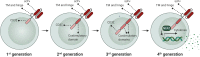
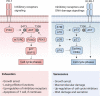


References
-
- Poorebrahim M, Abazari MF, Sadeghi S, Mahmoudi R, Kheirollahi A, Askari H, et al. Genetically modified immune cells targeting tumor antigens. Pharmacol Ther. 2020;214:107603.. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

