Proinflammatory IgG Fc structures in patients with severe COVID-19
- PMID: 33169014
- PMCID: PMC8130642
- DOI: 10.1038/s41590-020-00828-7
Proinflammatory IgG Fc structures in patients with severe COVID-19
Abstract
Severe acute respiratory syndrome coronavirus 2 infections can cause coronavirus disease 2019 (COVID-19), which manifests with a range of severities from mild illness to life-threatening pneumonia and multi-organ failure. Severe COVID-19 is characterized by an inflammatory signature, including high levels of inflammatory cytokines, alveolar inflammatory infiltrates and vascular microthrombi. Here we show that patients with severe COVID-19 produced a unique serologic signature, including an increased likelihood of IgG1 with afucosylated Fc glycans. This Fc modification on severe acute respiratory syndrome coronavirus 2 IgGs enhanced interactions with the activating Fcγ receptor FcγRIIIa; when incorporated into immune complexes, Fc afucosylation enhanced production of inflammatory cytokines by monocytes, including interleukin-6 and tumor necrosis factor. These results show that disease severity in COVID-19 correlates with the presence of proinflammatory IgG Fc structures, including afucosylated IgG1.
Figures


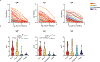

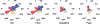
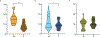

Update of
-
Proinflammatory IgG Fc structures in patients with severe COVID-19.medRxiv [Preprint]. 2020 Nov 10:2020.05.15.20103341. doi: 10.1101/2020.05.15.20103341. medRxiv. 2020. Update in: Nat Immunol. 2021 Jan;22(1):67-73. doi: 10.1038/s41590-020-00828-7. PMID: 32511463 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- R01AI139119/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- U19AI111825/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- R01 AI127877/AI/NIAID NIH HHS/United States
- P01 AI097092/AI/NIAID NIH HHS/United States
- U19 AI111825/AI/NIAID NIH HHS/United States
- R01 AI139119/AI/NIAID NIH HHS/United States
- R01 AI130398/AI/NIAID NIH HHS/United States
- U54CA260517/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)/International
- U54 CA260517/CA/NCI NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- R01 AI125567/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

