Laboratory Diagnosis and Monitoring the Viral Shedding of SARS-CoV-2 Infection
- PMID: 33169119
- PMCID: PMC7609236
- DOI: 10.1016/j.xinn.2020.100061
Laboratory Diagnosis and Monitoring the Viral Shedding of SARS-CoV-2 Infection
Abstract
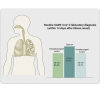
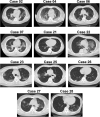
The worldwide epidemic of coronavirus disease 2019 (COVID-19) is ongoing. Rapid and accurate detection of the causative virus SARS-CoV-2 is vital for the treatment and control of COVID-19. In this study, the comparative sensitivity of different respiratory specimen types were retrospectively analyzed using 3,552 clinical samples from 410 COVID-19 patients confirmed by Guangdong CDC (Center for Disease Control and Prevention). Except for bronchoalveolar lavage fluid (BALF), the sputum possessed the highest positive rate (73.4%-87.5%), followed by nasal swabs (53.1%-85.3%) for both severe and mild cases during the first 14 days after illness onset (d.a.o.). Viral RNA could be detected in all BALF samples collected from the severe group within 14 d.a.o. and lasted up to 46 d.a.o. Moreover, although viral RNA was negative in the upper respiratory samples, it was also positive in BALF samples in most cases from the severe group during treatment. Notably, no viral RNA was detected in BALF samples from the mild group. Despite typical ground-glass opacity observed via computed tomographic scans, no viral RNA was detected in the first three or all upper respiratory tract specimens from some COVID-19 patients. In conclusion, sputum is most sensitive for routine laboratory diagnosis of COVID-19, followed by nasal swabs. Detection of viral RNA in BALF improves diagnostic accuracy in severe COVID-19 patients.
Keywords: COVID-19; SARS-CoV-2; molecular diagnosis; respiratory specimens; viral shedding.
© 2020.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

