Impact of COVID-19 Pandemic on the National PPM Tuberculosis Control Project in Korea: the Korean PPM Monitoring Database between July 2019 and June 2020
- PMID: 33169559
- PMCID: PMC7653169
- DOI: 10.3346/jkms.2020.35.e388
Impact of COVID-19 Pandemic on the National PPM Tuberculosis Control Project in Korea: the Korean PPM Monitoring Database between July 2019 and June 2020
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic caused disruptions to healthcare systems and endangered the control and prevention of tuberculosis (TB). We investigated the nationwide effects of COVID-19 on the national Public-Private Mix (PPM) TB control project in Korea, using monitoring indicators from the Korean PPM monitoring database.
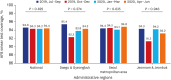
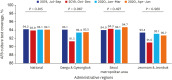
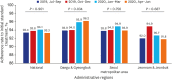
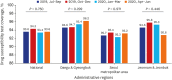
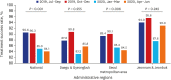
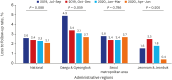
Methods: The Korean PPM monitoring database includes data from patients registered at PPM hospitals throughout the country. Data of six monitoring indicators for active TB cases updated between July 2019 and June 2020 were collected. The data of each cohort throughout the country and in Daegu-Gyeongbuk, Seoul Metropolitan Area, and Jeonnam-Jeonbuk were collated to provide nationwide data. The data were compared using the χ² test for trend to evaluate quarterly trends of each monitoring indicator at the national level and in the prespecified regions.
Results: Test coverages of sputum smear (P = 0.622) and culture (P = 0.815), drug susceptibility test (P = 0.750), and adherence rate to initial standard treatment (P = 0.901) at the national level were not significantly different during the study period. The rate of loss to follow-up among TB cases at the national level was not significantly different (P = 0.088); however, the treatment success rate among the smear-positive drug-susceptible pulmonary TB cohort at the national level significantly decreased, from 90.6% to 84.1% (P < 0.001). Treatment success rate in the Seoul metropolitan area also significantly decreased during the study period, from 89.4% to 84.5% (P = 0.006).
Conclusion: Our study showed that initial TB management during the COVID-19 pandemic was properly administered under the PPM project in Korea. However, our study cannot confirm or conclude a decreased treatment success rate after the COVID-19 pandemic due to limited data.
Keywords: Coronavirus; Public-private Sector Partnerships; Quality Indicators; SARS Virus; Treatment Outcome.
© 2020 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures
Similar articles
-
Conversations and Medical News Frames on Twitter: Infodemiological Study on COVID-19 in South Korea.J Med Internet Res. 2020 May 5;22(5):e18897. doi: 10.2196/18897. J Med Internet Res. 2020. PMID: 32325426 Free PMC article.
-
Tuberculosis Surveillance and Monitoring under the National Public-Private Mix Tuberculosis Control Project in South Korea 2016-2017.Tuberc Respir Dis (Seoul). 2020 Jul;83(3):218-227. doi: 10.4046/trd.2020.0016. Epub 2020 Jun 18. Tuberc Respir Dis (Seoul). 2020. PMID: 32610836 Free PMC article.
-
Two Pandemics, One Challenge-Leveraging Molecular Test Capacity of Tuberculosis Laboratories for Rapid COVID-19 Case-Finding.Emerg Infect Dis. 2020 Nov;26(11):2549-2554. doi: 10.3201/eid2611.202602. Epub 2020 Sep 21. Emerg Infect Dis. 2020. PMID: 32956612 Free PMC article.
-
Systematic Review of Clinical Insights into Novel Coronavirus (CoVID-19) Pandemic: Persisting Challenges in U.S. Rural Population.Int J Environ Res Public Health. 2020 Jun 15;17(12):4279. doi: 10.3390/ijerph17124279. Int J Environ Res Public Health. 2020. PMID: 32549334 Free PMC article.
-
Management of the COVID-19 Pandemic in the Republic of Korea from the Perspective of Governance and Public-Private Partnership.Yonsei Med J. 2021 Sep;62(9):777-791. doi: 10.3349/ymj.2021.62.9.777. Yonsei Med J. 2021. PMID: 34427063 Free PMC article. Review.
Cited by
-
Retrospective Cohort Study of Effects of the COVID-19 Pandemic on Tuberculosis Notifications, Vietnam, 2020.Emerg Infect Dis. 2022 Mar;28(3):684-692. doi: 10.3201/eid2803.211919. Emerg Infect Dis. 2022. PMID: 35202526 Free PMC article.
-
Trends of legionellosis reported in Jeju Province, Republic of Korea, 2015-2022.Osong Public Health Res Perspect. 2023 Aug;14(4):321-327. doi: 10.24171/j.phrp.2023.0145. Epub 2023 Aug 21. Osong Public Health Res Perspect. 2023. PMID: 37652687 Free PMC article.
-
Delays in the diagnosis and treatment of tuberculosis during the COVID-19 outbreak in the Republic of Korea in 2020.Osong Public Health Res Perspect. 2021 Oct;12(5):293-303. doi: 10.24171/j.phrp.2021.0063. Epub 2021 Sep 23. Osong Public Health Res Perspect. 2021. PMID: 34719221 Free PMC article.
-
Impact of the Coronavirus Disease Pandemic on Patients with Head Injuries in South Korea.J Korean Neurosurg Soc. 2022 Mar;65(2):269-275. doi: 10.3340/jkns.2021.0076. Epub 2022 Feb 3. J Korean Neurosurg Soc. 2022. PMID: 35108772 Free PMC article.
-
Impact of COVID-19 Pandemic on Tuberculosis Preventive Services and Their Post-Pandemic Recovery Strategies: A Rapid Review of Literature.J Korean Med Sci. 2023 Feb 6;38(5):e43. doi: 10.3346/jkms.2023.38.e43. J Korean Med Sci. 2023. PMID: 36747365 Free PMC article. Review.
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-51 (11 March 2020) [Accessed October 27, 2020]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
-
- Oh J, Lee JK, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020;6(1):e1753464. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

