Zika and Dengue Interactions in the Context of a Large Dengue Vaccine Clinical Trial in Latin America
- PMID: 33169661
- PMCID: PMC7790115
- DOI: 10.4269/ajtmh.20-0635
Zika and Dengue Interactions in the Context of a Large Dengue Vaccine Clinical Trial in Latin America
Abstract
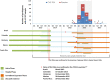
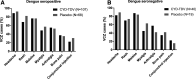
A phase III dengue vaccine trial including 9- to 16-year-olds in Latin America (NCT01374516) was ongoing at the time of a Zika outbreak. We explored interactions between dengue and Zika, in the context of dengue vaccination. Symptomatic virologically confirmed Zika (VCZ) was evaluated using acute-phase sera from febrile participants (January 2013-March 2018). Neutralizing antibody geometric mean titers (GMTs) were evaluated pre- and post-Zika outbreak (months 25 and 72) in 2,000 randomly selected participants. Baseline dengue serostatus was determined using the plaque reduction neutralization test or inferred post hoc using nonstructural protein 1 IgG ELISA at M13 (case-cohort analysis). Vaccine efficacy against VCZ and serologically suspected Zika (SSZ) was estimated. Overall, 239/10,157 (2.4%) acute-phase samples were VCZ positive during the study. Dengue vaccine efficacy against VCZ was 27.8% (95% CI: 0.3; 47.7) among baseline dengue-seropositive participants. No vaccine effect was evident against SSZ. Zika antibody GMTs increased from pre- to post-Zika epidemic, with smaller increases observed for participants who were dengue seropositive at baseline than for those who were dengue seronegative: post-/pre-Zika GMT ratios for baseline dengue-seropositive participants were 21.5 (vaccine group) and 30.8 (placebo); and for dengue seronegatives, 88.1 and 89.5, respectively. Dengue antibody GMTs post-Zika were higher in dengue vaccine and placebo recipients with SSZ than those without SSZ in both dengue seropositives and seronegatives. Dengue vaccine did not enhance symptomatic Zika illness in dengue-seropositive individuals, rather it reduced the risk of VCZ. Zika infection boosted preexisting vaccine-induced or naturally occurring dengue-neutralizing antibodies.
Conflict of interest statement
Disclosures: B. Z., G. H. D., C. A. D., F. N., E. L., M.I. B., M. C., S. S., and Y. W. are Sanofi Pasteur fulltime employees. B. Z., G. H. D., C. A. D., E. L., F. N., M. I. B., M. C., and S. S. hold Sanofi shares/stock options. H. R. implements clinical trials for various pharmaceutical companies including Sanofi Pasteur. C. D., K. L., D. M. R., H. R., and J. L. A. received funding from Sanofi Pasteur to support their work on the CYD15 trial.
Data Sharing Statement: Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at:
Figures




References
-
- Zambrano B, San Martin JL, 2014. Epidemiology of dengue in Latin America. J Pediatric Infect Dis Soc 3: 181–182. - PubMed
-
- World Health Organization , 2019. Dengue and Severe Dengue. WHO factsheet. Geneva, Switzerland: WHO; Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue. Accessed January 21, 2020.
-
- Petersen LR, Jamieson DJ, Powers AM, Honein MA, 2016. Zika virus. N Engl J Med 374: 1552–1563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

