Intravenous versus intramuscular prophylactic oxytocin for the third stage of labour
- PMID: 33169839
- PMCID: PMC8236306
- DOI: 10.1002/14651858.CD009332.pub4
Intravenous versus intramuscular prophylactic oxytocin for the third stage of labour
Abstract
Background: There is general agreement that oxytocin given either through the intravenous or intramuscular route is effective in reducing postpartum blood loss. However, it is unclear whether the subtle differences between the mode of action of these routes have any effect on maternal and infant outcomes. This review was first published in 2012 and last updated in 2018.
Objectives: To determine the comparative effectiveness and safety of oxytocin administered intravenously or intramuscularly for prophylactic management of the third stage of labour after vaginal birth.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (19 December 2019), and reference lists of retrieved studies.
Selection criteria: Eligible studies were randomised trials comparing intravenous with intramuscular oxytocin for prophylactic management of the third stage of labour after vaginal birth. We excluded quasi-randomised trials.
Data collection and analysis: Two review authors independently assessed studies for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed the certainty of the evidence with the GRADE approach.
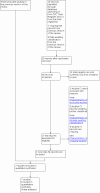
Main results: Seven trials, involving 7817 women, met the inclusion criteria for this review. The trials compared intravenous versus intramuscular administration of oxytocin just after the birth of the anterior shoulder or soon after the birth of the baby. All trials were conducted in hospital settings and included women with term pregnancies, undergoing a vaginal birth. Overall, the included studies were at moderate or low risk of bias, with two trials providing clear information on allocation concealment and blinding. For GRADE outcomes, the certainty of the evidence was generally moderate to high, except from two cases where the certainty of the evidence was either low or very low. High-certainty evidence suggests that intravenous administration of oxytocin in the third stage of labour compared with intramuscular administration carries a lower risk for postpartum haemorrhage (PPH) ≥ 500 mL (average risk ratio (RR) 0.78, 95% confidence interval (CI) 0.66 to 0.92; six trials; 7731 women) and blood transfusion (average RR 0.44, 95% CI 0.26 to 0.77; four trials; 6684 women). Intravenous administration of oxytocin probably reduces the risk of PPH ≥ 1000 mL, although the 95% CI crosses the line of no-effect (average RR 0.65, 95% CI 0.39 to 1.08; four trials; 6681 women; moderate-certainty evidence). In all studies but one, there was a reduction in the risk of PPH ≥ 1000 mL with intravenous oxytocin. The study that found a large increase with intravenous administration was small (256 women), and contributed only 3% of total events. Once this small study was removed from the meta-analysis, heterogeneity was eliminated and the treatment effect favoured intravenous oxytocin (average RR 0.61, 95% CI 0.42 to 0.88; three trials; 6425 women; high-certainty evidence). Additionally, a sensitivity analysis, exploring the effect of risk of bias by restricting analysis to those studies rated as 'low risk of bias' for random sequence generation and allocation concealment, found that the prophylactic administration of intravenous oxytocin reduces the risk for PPH ≥ 1000 mL, compared with intramuscular oxytocin (average RR 0.64, 95% CI 0.43 to 0.94; two trials; 1512 women). The two routes of oxytocin administration may be comparable in terms of additional uterotonic use (average RR 0.78, 95% CI 0.49 to 1.25; six trials; 7327 women; low-certainty evidence). Although intravenous compared with intramuscular administration of oxytocin probably results in a lower risk for serious maternal morbidity (e.g. hysterectomy, organ failure, coma, intensive care unit admissions), the confidence interval suggests a substantial reduction, but also touches the line of no-effect. This suggests that there may be no reduction in serious maternal morbidity (average RR 0.47, 95% CI 0.22 to 1.00; four trials; 7028 women; moderate-certainty evidence). Most events occurred in one study from Ireland reporting high dependency unit admissions, whereas in the remaining three studies there was only one case of uvular oedema. There were no maternal deaths reported in any of the included studies (very low-certainty evidence). There is probably little or no difference in the risk of hypotension between intravenous and intramuscular administration of oxytocin (RR 1.01, 95% CI 0.88 to 1.15; four trials; 6468 women; moderate-certainty evidence). Subgroup analyses based on the mode of administration of intravenous oxytocin (bolus injection or infusion) versus intramuscular oxytocin did not show any substantial differences on the primary outcomes. Similarly, additional subgroup analyses based on whether oxytocin was used alone or as part of active management of the third stage of labour (AMTSL) did not show any substantial differences between the two routes of administration.
Authors' conclusions: Intravenous administration of oxytocin is more effective than its intramuscular administration in preventing PPH during vaginal birth. Intravenous oxytocin administration presents no additional safety concerns and has a comparable side effects profile with its intramuscular administration. Future studies should consider the acceptability, feasibility and resource use for the intervention, especially in low-resource settings.
Trial registration: ClinicalTrials.gov NCT01914419 NCT02080104 NCT02954068 NCT01954186 NCT00200252 NCT02319707 NCT01608958.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
OT Oladapo: the first version of this review was performed under a contractual Agreement for Performance of Work (APW) between WHO and the contact author. No funding was allocated for the preparation of this update. However, the contact author is currently a paid staff member of the WHO.
BO Okusanya: none known.
E Abalos: none known.
ID Gallos: none known.
A Papadopoulou: none known.
Figures


































Update of
-
Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour.Cochrane Database Syst Rev. 2018 Sep 22;9(9):CD009332. doi: 10.1002/14651858.CD009332.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Nov 9;11:CD009332. doi: 10.1002/14651858.CD009332.pub4. PMID: 30246877 Free PMC article. Updated.
References
References to studies included in this review
Adnan 2018 {published data only}ISRCTN14718882
-
- ISRCTN14718882. A study to compare the effectiveness of intravenous oxytocin with intramuscular oxytocin given at the third stage of labour at preventing bleeding at vaginal birth. isrctn.com/ISRCTN14718882 (first received 14 December 2015).
Charles 2019 {published data only}
-
- Charles D, Anger H, Dabash R, Darwish E, Ramadan MC, Mansy A, et al. Intramuscular injection, intravenous infusion, and intravenous bolus of oxytocin in the third stage of labor for prevention of postpartum hemorrhage: a three-arm randomized control trial. BMC Pregnancy and Childbirth 2019;19(1):38. [CENTRAL: CN-01788660] [EMBASE: 626012794] [PMID: ] - PMC - PubMed
-
- NCT01914419. Administration of oxytocin via intramuscular injection and intravenous bolus or intravenous infusion in the third stage of labor for prevention of postpartum hemorrhage. clinicaltrials.gov/show/NCT01914419 (first received 31 July 2013).
Dagdeviren 2016 {published data only}
-
- Dagdeviren H, Cengiz H, Heydarova U, Caypinar SS, Kanawati A, Guven E, et al. Intramuscular versus intravenous prophylactic oxytocin for postpartum hemorrhage after vaginal delivery: a randomized controlled study. Archives of Gynecology and Obstetrics 2016;294(5):911-6. - PubMed
-
- Dagdeviren H. Intramuscular versus intravenous prophylactic oxytocin for hemorrhage after vaginal delivery (oxytocin). clinicaltrials.gov/ct2/show/NCT02080104 (first received 1 March 2014).
Durocher 2019 {published data only}
-
- Carroli G, Durocher J, Dzuba I, Morales E, Aguirre D, Esquivel J, et al. Does route matter? intravenous versus intramuscular oxytocin for prevention of postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2018;143(Suppl 3):236. [CENTRAL: CN-01654052] [EMBASE: 624607003]
-
- Durocher J, Dzuba IG, Martin R, Winikoff B, Carroli G, Carroli B, et al. Does route matter? Impact of route of oxytocin administration on postpartum bleeding: a double-blind, randomized controlled trial. PloS One 2019;14(10):e0222981. [CENTRAL: CN-01996457] [EMBASE: 2003128926] [PMID: ] - PMC - PubMed
-
- NCT02954068. Intravenous versus intramuscular administration of oxytocin and its relationship with postpartum bleeding and other clinical signs: a randomized placebo-controlled study. clinicaltrials.gov/show/NCT02954068 (first received 28 October 2016).
Neri‐Mejia 2016 {published data only}
-
- Neri-Mejia M, Pedraza-Aviles AG. Active management of the third stage of labor: three schemes of oxytocin: randomised clinical trial. Ginecologia y Obstetricia De Mexico 2016;84(5):306-13. - PubMed
Oguz 2014 {published data only}
-
- NCT01954186. When and how to administer oxytocin for active management of third stage of labour. clinicaltrials.gov/show/NCT01954186 (first received 22 September 2013).
-
- Oguz Orhan E, Dilbaz B, Aksakal SE, Altinbas S, Erkaya S. Prospective randomized trial of oxytocin administration for active management of the third stage of labor. International Journal of Gynecology and Obstetrics 2014;127(2):175-9. - PubMed
Sangkhomkhamhang 2015 {published data only}
-
- ACTRN12612000624886. A randomised controlled trial of intravenous versus intramuscular oxytocin in the management of third stage of labor. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000624886 (first received 12 June 2012).
-
- Sangkomkamhang U, Kruangpatee. A randomized controlled trial of intravenous versus intramuscular oxytocin in the prevention of postpartum hemorrhage during the third stage of labor. Journal of Health Science 2015;24(2):354-9.
References to studies excluded from this review
NCT03651882 {published data only}
-
- NCT03651882. Oxytocin i.m./i.v. versus carbetocin i.v. in elective cesarean sections. Https://clinicaltrials.gov/show/nct03651882 2018.
Sheldon 2011 {published and unpublished data}
-
- Durocher J, Blum J, Sheldon WR, Trussell J, Winikoff B. Does the effect of oxytocin prophylaxis on post-partum blood loss depend on route of administration? International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S332.
-
- Sheldon W. Gynuity Health Projects: assessing effectiveness of the AMTSL components. Personal communication July 2011.
References to studies awaiting assessment
Ashwal 2016 {published data only}
-
- Ashwal E, Hiersch L, Wertheimer A, Krispin E, Aviram A, Dayan DB, et al. The effect of post-partum oxytocin regimen on hemoglobin decline–a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S197-S198, Abstract no: 351.
NCT00200252 {published data only}
-
- NCT00200252. Intramuscular versus intravascular oxytocin for the third stage of labour. clinicaltrials.gov/ct2/show/record/NCT00200252 (first received 12 September 2015).
NCT02319707 {published data only}
-
- NCT02319707. Management of the third stage of labor. clinicaltrials.gov/ct2/show/NCT02319707 (first received 14 December 2014).
References to ongoing studies
ACTRN12617000176369 {published data only}
-
- ACTRN12617000176369. Correlative assessment of prophylactic oxytocin pharmacokinetics (PK) to maternal body mass index (BMI) following intravenous (IV) and intramuscular (IM) administration, for the prevention of postpartum haemorrhage, at elective caesarean section. An open label, prospective, exploratory, randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617000176369 (first received 23 January 2017).
NCT01608958 {published data only}
-
- Dzuba I, Durocher J, Dilbaz B, Gelisen O, Ngoc NTN, Montesinos R, et al. Route of administration of oxytocin in prevention of postpartum hemorrhage. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S333.
-
- NCT01608958. Intravenous and intramuscular administration of oxytocin in the third stage of labor for prevention of postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT01608958 (first received 29 May 2012).
PACTR201902721929705 {published data only}
-
- PACTR201902721929705. A double-blind randomized trial on comparison of intravenous and intramuscular oxytocin for preventing atonic primary post partum haemorrhage in third stage of labour. https://pactr.samrc.ac.za/trialdisplay.aspx? Trialid=5920 2019.
Additional references
Begley 2019
Blum 2010
-
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post-partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet 2010;375(9710):217-23. - PubMed
Bolton 2003
-
- Bolton TJ, Randall K, Yentis SM. Effect of the confidential enquiries into maternal deaths on the use of syntocinon at caesarean section in the UK. Anaesthesia 2003;58:277-9. - PubMed
Breathnach 2006
-
- Breathnach F, Geary M. Standard medical therapy. In: B-Lynch C, Keith LG, Lalonde AB, Karoshi M, editors(s). A textbook of postpartum haemorrhage. United Kingdom: Sapiens publishing, 2006:256-62.
Carroli 2008
-
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics and Gynaecology 2008;22:999–1012. - PubMed
Carroll 2016
Charbit 2004
-
- Charbit B, Funck-Brentano C, Samain E, Jannier-Guillou V, Albaladejo P, Marty J. QT interval prolongation after oxytocin bolus during surgical induced abortion. Clinical Pharmacology and Therapeutics 2007;76(4):359-64. - PubMed
Cooper 2002
-
- Cooper GM, Lewis G, Neilson J. Confidential enquiries into maternal deaths, 1997-1999. British Journal of Anaesthesia 2002;89(4):369-72. [DOI: ] - PubMed
Dale 1909
Davies 2005
-
- Davies GA, Tessier JL, Woodman MC, Lipson A, Hahn PM. Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2011;105(2):294-9. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JPT, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Du Vigneaud 1954
-
- Du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG, Gordon S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. Journal of the American Chemical Society 1954;75(19):4879-80.
FIGO 2012
-
- FIGO Safe Motherhood and Newborn Health (SMNH) Committee. Prevention and treatment of postpartum hemorrhage in low-resource settings. International Journal of Gynecology and Obstetrics 2012;117:108-18. - PubMed
Gallos 2018
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT. Version 'date accessed' 30/10/17. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hendricks 1970
-
- Hendricks CH, Brenner WE. Cardiovascular effects of oxytocic drugs used post partum. American Journal of Obstetrics and Gynecology 1970;108(5):751-60. - PubMed
Higgins 2003
Higgins 2019
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hutin 2003
In 2011
Langesaeter 2009
-
- Langesaeter E, Rosseland LA, Stubhaug A. Haemodynamic effects of repeated doses of oxytocin during caesarean delivery in healthy parturients. British Journal of Anaesthesia 2009;103(2):260-2. - PubMed
Lazarus 2005
-
- Lazarus JV, Lalonde A. Reducing postpartum haemorrhage in Africa. International Journal of Gynecology & Obstetrics 2005;88(1):89-90. - PubMed
NICE 2014
-
- National Institute for Health and Care Excellence (NICE). Intrapartum care for healthy women and babies; Clinical guideline 190. www.nice.org.uk (accessed online on 4 April 2020).
Oladapo 2016
-
- Oladapo OT, Adetoro OO, Ekele BA, Chama C, Etuk SJ, Aboyeji AP, on behalf of the Nigeria near-miss and maternal death surveillance network. When getting there is not enough: a nationwide cross-sectional study of 998 maternal deaths and 1451 near-misses in public tertiary hospitals in a low-income country. BJOG: an international journal of obstetrics and gynaecology 2016;123:928-38. [DOI: 10.1111/1471-0528.13450] - DOI - PMC - PubMed
Pinder 2002
-
- Pinder AJ, Dresner M, Calow C, Shorten GD, O'Riordan J, Johnson R. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. International Journal of Obstetric Anesthesia 2002;11(3):156-9. - PubMed
Prendiville 2000
RevMan 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salati 2019
Say 2014
-
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323-333. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. GRADE Working Group. Updated October 2013. Available from: gdt.gradepro.org/app/handbook/handbook.html.
Secher 1978
-
- Secher NJ, Arnsbo P, Wallin L. Haemodynamic effects of oxytocin (syntocinon) and methyl ergometrine (methergin) on the systemic and pulmonary circulations of pregnant anaesthetized women. Acta Obstetricia et Gynecologica Scandinavica 1978;57(2):97-103. - PubMed
Soltani 2010
Souza 2013
-
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet 2013;381:1747-55. - PubMed
Spence 2002
-
- Spence A. Oxytocin during caesarean section. Anaesthesia 2002;57:722–3. - PubMed
Sterne 2017
-
- Sterne JAC, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Strand 2005
-
- Strand RT, Da Silva F, Jangsten E, Bergström S. Postpartum hemorrhage: a prospective, comparative study in Angola using a new disposable device for oxytocin administration. Acta Obstetricia et Gynecologica Scandinavica 2005;84(3):260-5. - PubMed
Su 2012
Svanström 2008
-
- Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during caesarean section. British Journal of Anaesthesia 2008;100(5):683-9. - PubMed
Thomas 2007
-
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. British Journal of Anaesthesia 2007;98(1):116-9. - PubMed
Tsu 2003
-
- Tsu VD, Sutanto A, Vaidya K, Coffey P, Widjaya A. Oxytocin in prefilled Uniject injection devices for managing third-stage labor in Indonesia. International Journal of Gynecology & Obstetrics 2003;83(1):103-11. - PubMed
Tsu 2009
-
- Tsu VD, Luu HT, Mai TT. Does a novel prefilled injection device make postpartum oxytocin easier to administer? Results from midwives in Vietnam. Midwifery 2009;25(4):461-5. - PubMed
Van Loon 2020
-
- Van Loon FHJ, Leggett T, Bouwman ARA, Dierick-van Daele ATM. Cost-utilization of peripheral intravenous cannulation in hospitalized adults: An observational study. The Journal of Vascular Access 2020;Epub-ahead of print. - PubMed
Weis 1975
-
- Weis FR Jr, Markello R, Mo B, Bochiechio P. Cardiovascular effects of oxytocin. Obstetrics & Gynecology 1975;46:211-4. - PubMed
WHO 2012
-
- World Health Organization (WHO). WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization, 2012. - PubMed
WHO 2017
-
- World Health Organization (WHO). Managing complications in pregnancy and childbirth: a guide for midwives and doctors. 2nd edition. Geneva: World Health Organization, 2017.
WHO 2018
-
- World Health Organization (WHO). WHO recommendations: Uterotonics for the prevention of postpartum haemorrhage. Geneva: World Health Organization, 2018. - PubMed
WHO 2019
-
- World Health Organization (WHO). Trends in maternal mortality:2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. https://www.who.int/reproductivehealth/publications/maternal-mortality-2... (accessed 20 January 2020).
References to other published versions of this review
Oladapo 2012
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

