Unknown cytomegalovirus serostatus in primary immunodeficiency disorders: A new category of transplant recipients
- PMID: 33169931
- PMCID: PMC8287897
- DOI: 10.1111/tid.13504
Unknown cytomegalovirus serostatus in primary immunodeficiency disorders: A new category of transplant recipients
Abstract
Background: Cytomegalovirus (CMV) serostatus of recipient (R) and donor (D) influences hematopoietic stem cell transplant (HSCT) outcome. However, it is not a reliable indicator of CMV infection in primary immunodeficiency disorder (PIDD) recipients who are unable to make adequate antigen-specific immunoglobulin (Ig) or who receive intravenous Ig (IVIg) prior to testing.
Objective: Since no data exist on PIDD with unknown CMV serostatus, we aimed to evaluate the relationship between pre-HSCT recipient and donor serostatus and incidence of CMV infection in recipients with unknown serostatus.
Methods: A retrospective analysis of all pediatric PIDD HSCTs (2007-2018) was performed at University of California San Francisco. Recipients were separated into categories based on pre-transplant serostatus: 1) seropositive (R(+)), 2) seronegative (R(-)), and 3) unknown serostatus (R(x)). Patients with pre-HSCT active CMV viremia were excluded.
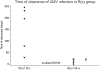
Results: A total of 90 patients were included, 69% male. The overall incidence of CMV infection was 20%, but varied in R(+), R(-), and R(x) at 80%, 0%, and 14%, (P-value = .0001). Similarly, 5-year survival differed among groups, 60% R(+), 100% R(-), and 90% of R(x) (P-value = .0045). There was no difference in CMV reactivation by donor serostatus (P-value = .29), however, faster time to clearance of CMV was observed for R(x)/D(+) group (median 9.5 days (IQR 2.5-18), P-value = .024).
Conclusion: We identify a novel group of recipients, R(x), with an intermediate level of survival and CMV incidence post-HSCT, when compared to seropositive and seronegative recipients. No evidence of CMV transmission from D(+) in R(-) and R(x) was observed. We believe R(x) should be considered as a separate category in future studies to better delineate recipient risk status.
Keywords: Cytomegalovirus; hematopoietic stem cell transplant; infection; primary immunodeficiency; serostatus.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
Figures


References
-
- van der Heiden P, Marijt E, Falkenburg F, Jedema I. Control of cytomegalovirus viremia after allogeneic stem cell transplantation: a review on CMV-Specific T cell reconstitution. Biol Blood Marrow Transplant. 2018;24(9):1776–1782. - PubMed
-
- Emery V, Zuckerman M, Jackson G, et al. Management of cytomegalovirus infection in haemopoietic stem cell transplantation. Br J Haematol. 2013;162(1):25–39. - PubMed
-
- George B, Pati N, Gilroy N, et al. Pre-transplant cytomegalovirus (CMV ) serostatus remains the most impor tant determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12(4):322–329. - PubMed

