Effects of TM6SF2 E167K on hepatic lipid and very low-density lipoprotein metabolism in humans
- PMID: 33170809
- PMCID: PMC7819740
- DOI: 10.1172/jci.insight.144079
Effects of TM6SF2 E167K on hepatic lipid and very low-density lipoprotein metabolism in humans
Abstract
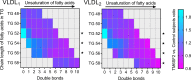
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic lipid accumulation. The transmembrane 6 superfamily member 2 (TM6SF2) E167K genetic variant associates with NAFLD and with reduced plasma triglyceride levels in humans. However, the molecular mechanisms underlying these associations remain unclear. We hypothesized that TM6SF2 E167K affects hepatic very low-density lipoprotein (VLDL) secretion and studied the kinetics of apolipoprotein B100 (apoB100) and triglyceride metabolism in VLDL in homozygous subjects. In 10 homozygote TM6SF2 E167K carriers and 10 matched controls, we employed stable-isotope tracer and compartmental modeling techniques to determine apoB100 and triglyceride kinetics in the 2 major VLDL subfractions: large triglyceride-rich VLDL1 and smaller, less triglyceride-rich VLDL2. VLDL1-apoB100 production was markedly reduced in homozygote TM6SF2 E167K carriers compared with controls. Likewise, VLDL1-triglyceride production was 35% lower in the TM6SF2 E167K carriers. In contrast, the direct production rates for VLDL2-apoB100 and triglyceride were not different between carriers and controls. In conclusion, the TM6SF2 E167K genetic variant was linked to a specific reduction in hepatic secretion of large triglyceride-rich VLDL1. The impaired secretion of VLDL1 explains the reduced plasma triglyceride concentration and provides a basis for understanding the lower risk of cardiovascular disease associated with the TM6SF2 E167K genetic variant.
Keywords: Hepatology; Lipoproteins; Metabolism.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

