A novel role for kynurenine 3-monooxygenase in mitochondrial dynamics
- PMID: 33170836
- PMCID: PMC7654755
- DOI: 10.1371/journal.pgen.1009129
A novel role for kynurenine 3-monooxygenase in mitochondrial dynamics
Abstract
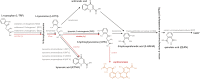
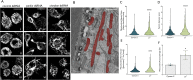
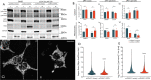
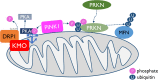
The enzyme kynurenine 3-monooxygenase (KMO) operates at a critical branch-point in the kynurenine pathway (KP), the major route of tryptophan metabolism. As the KP has been implicated in the pathogenesis of several human diseases, KMO and other enzymes that control metabolic flux through the pathway are potential therapeutic targets for these disorders. While KMO is localized to the outer mitochondrial membrane in eukaryotic organisms, no mitochondrial role for KMO has been described. In this study, KMO deficient Drosophila melanogaster were investigated for mitochondrial phenotypes in vitro and in vivo. We find that a loss of function allele or RNAi knockdown of the Drosophila KMO ortholog (cinnabar) causes a range of morphological and functional alterations to mitochondria, which are independent of changes to levels of KP metabolites. Notably, cinnabar genetically interacts with the Parkinson's disease associated genes Pink1 and parkin, as well as the mitochondrial fission gene Drp1, implicating KMO in mitochondrial dynamics and mitophagy, mechanisms which govern the maintenance of a healthy mitochondrial network. Overexpression of human KMO in mammalian cells finds that KMO plays a role in the post-translational regulation of DRP1. These findings reveal a novel mitochondrial role for KMO, independent from its enzymatic role in the kynurenine pathway.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: FG has a patent application - KYNURENINE 3-MONOOXYGENASE (KMO) INHIBITORS, AND USES AND COMPOSITIONS THEREOF – pending. The authors have also received research support for this study as described in the financial disclosure.
Figures




References
-
- Breda C, Sathyasaikumar KV, Sograte Idrissi S, Notarangelo FM, Estranero JG, Moore GGL, et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci USA. 2016;113:5435–5440. 10.1073/pnas.1604453113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

