Efficacy and safety of dolutegravir plus emtricitabine versus standard ART for the maintenance of HIV-1 suppression: 48-week results of the factorial, randomized, non-inferiority SIMPL'HIV trial
- PMID: 33170863
- PMCID: PMC7654764
- DOI: 10.1371/journal.pmed.1003421
Efficacy and safety of dolutegravir plus emtricitabine versus standard ART for the maintenance of HIV-1 suppression: 48-week results of the factorial, randomized, non-inferiority SIMPL'HIV trial
Abstract
Background: Dolutegravir (DTG)-based dual therapy is becoming a new paradigm for both the initiation and maintenance of HIV treatment. The SIMPL'HIV study investigated the outcomes of virologically suppressed patients on standard combination antiretroviral therapy (cART) switching to DTG + emtricitabine (FTC). We present the 48-week efficacy and safety data on DTG + FTC versus cART.
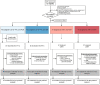
Methods and findings: SIMPL'HIV was a multicenter, open-label, non-inferiority randomized trial with a factorial design among treatment-experienced people with HIV in Switzerland. Participants were enrolled between 12 May 2017 and 30 May 2018. Patients virologically suppressed for at least 24 weeks on standard cART were randomized 1:1 to switching to DTG + FTC or to continuing cART, and 1:1 to simplified patient-centered monitoring versus standard monitoring. The primary endpoint was the proportion of patients virologically suppressed with <100 copies/ml through 48 weeks. The secondary endpoints included virological suppression at 48 weeks according to the US Food and Drug Administration (FDA) snapshot analysis. Non-inferiority of DTG + FTC versus cART for viral suppression was assessed using a stratified Mantel-Haenszel risk difference, with non-inferiority declared if the lower bound of the 95% confidence interval was greater than -12%. Adverse events were monitored to assess safety. Quality of life was evaluated using the PROQOL-HIV questionnaire. Ninety-three participants were randomized to DTG + FTC, and 94 individuals to cART. Median nadir CD4 count was 246 cells/mm3; median age was 48 years; 17% of participants were female. DTG + FTC was non-inferior to cART. The proportion of patients with viral suppression (<100 copies/ml) through 48 weeks was 93.5% in the DTG + FTC arm and 94.7% in the cART arm in the intention-to-treat population (risk difference -1.2%; 95% CI -7.8% to 5.6%). Per-protocol analysis showed similar results, with viral suppression in 96.5% of patients in both arms (risk difference 0.0%; 95% CI -5.6% to 5.5%). There was no relevant interaction between the type of treatment and monitoring (interaction ratio 0.98; 95% CI 0.85 to 1.13; p = 0.81). Using the FDA snapshot algorithm, 84/93 (90.3%) participants in the DTG + FTC arm had an HIV-1 RNA viral load of <50 copies/ml compared to 86/94 (91.5%) participants on standard cART (risk difference -1.1%; 95% CI -9.3% to 7.1%; p = 0.791). The overall proportion of patients with adverse events and discontinuations did not differ by randomization arm. The proportion of patients with serious adverse events was higher in the cART arm (16%) compared to the DTG + FTC arm (6.5%) (p = 0.041), but none was considered to be related to the study medication. Quality of life improved more between baseline and week 48 in the DTG + FTC compared to the cART arm (adjusted difference +2.6; 95% CI +0.4 to +4.7). The study's main limitations included a rather small proportion of women included, the open label design, and its short duration.
Conclusions: In this study, DTG + FTC as maintenance therapy was non-inferior to cART in terms of efficacy, with a similar safety profile and a greater improvement in quality of life, thus expanding the offer of 2-drug simplification options among virologically suppressed individuals.
Trial registration: ClinicalTrials.gov NCT03160105.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: KJM has received travel grants and honoraria from Gilead Sciences, Roche Diagnostics, GlaxoSmithKline, Merck Sharp & Dohme, Bristol-Myers Squibb, ViiV and Abbott; and the University of Zurich received research grants from Gilead Science, Roche, and Merck Sharp & Dohme for studies that Dr Metzner serves as principal investigator, and advisory board honoraria from Gilead Sciences. AC is a member of the WHO guidelines panel (GSG) from 2017 up to 2020. The HIV Unit of Geneva University Hospitals (HUG) received unrestricted educational grants from ViiV, AbbVie, Gilead and MSD; ViiV, Gilead and MSD also provided financial support to the LIPO group and metabolism (day hospital) in the HIV/AIDS Unit of HUG. HFG has received unrestricted Research Grants from Gilead Sciences and Roche; fees for data and safety monitoring board membership from Merck; consulting/advisory board membership fees from Gilead Sciences, ViiV, Merck, Sandoz and Mepha. MS has received advisory board honoraria (paid to the institution) from Gilead, MSD Janssen, ViiV, Sandoz and Mepha and travel/conference Grants from Gilead and MSD. MC institution received Research Grants from Gilead and Viiv, and expert opinion’s fees from Abbvie, Gilead, MSD, Viiv and Sandoz. DLB has received honoraria and travel grants from Merck, Gilead and ViiV. GW has received research grants from Gilead Sciences and travel grants/honoraria from Gilead Sciences, AbbVie and ViiV, (paid to the institution). ME is a member of the Editorial Board of Plos Medicine. DS, AM, MB, ME, SY, LD, AL, PS, EB and PV have no competing of interests to declare.
Figures

References
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Washington (DC): Department of Health and Human Services; 2020. [cited 2020 Oct 16]. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/Ad....
-
- European AIDS Clinical Society. EACS guidelines 2020. Version 10.1. Brussels: European AIDS Clinical Society; 2020. [cited 2020 Oct 20]. https://eacs.sanfordguide.com/front-page.
-
- Baril JG, Angel JB, Gill MJ, Gathe J, Cahn P, van Wyk J, et al. Dual therapy treatment strategies for the management of patients infected with HIV: a systematic review of current evidence in ARV-naive or ARV-experienced, virologically suppressed patients. PLoS ONE. 2016;11(2):e0148231 10.1371/journal.pone.0148231 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

