Immune thrombocytopenia (ITP) World Impact Survey (iWISh): Patient and physician perceptions of diagnosis, signs and symptoms, and treatment
- PMID: 33170956
- PMCID: PMC7898610
- DOI: 10.1002/ajh.26045
Immune thrombocytopenia (ITP) World Impact Survey (iWISh): Patient and physician perceptions of diagnosis, signs and symptoms, and treatment
Erratum in
-
CORRIGENDUM.Am J Hematol. 2021 Oct 1;96(10):1343. doi: 10.1002/ajh.26139. Epub 2021 Mar 1. Am J Hematol. 2021. PMID: 34494302 Free PMC article. No abstract available.
Abstract
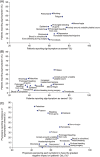
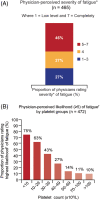
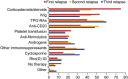
Immune thrombocytopenia (ITP) is now well-known to reduce patients' health-related quality of life. However, data describing which signs and symptoms patients and physicians perceive as having the greatest impact are limited, as is understanding the full effects of ITP treatments. I-WISh (ITP World Impact Survey) was an exploratory, cross-sectional survey designed to establish the multifaceted impact of ITP, and its treatments, on patients' lives. It focused on perceptions of 1507 patients and 472 physicians from 13 countries regarding diagnostic pathway, frequency and severity of signs and symptoms, and treatment use. Twenty-two percent of patients experienced delayed diagnosis (caused by several factors), 73% of whom felt anxious as a result. Patients rated fatigue among the most frequent, severe symptom associated with ITP at diagnosis (58% most frequent; 73% most severe), although physicians assigned it lower priority (30%). Fatigue was one of the few symptoms persisting at survey completion (50% and 65%, respectively) and was the top symptom patients wanted resolved (46%). Participating physicians were experienced at treating ITP, thereby recognizing the need to limit corticosteroid use to newly-diagnosed or first-relapse patients and espoused increased use of thrombopoietin receptor agonists and anti-CD20 after relapse in patients with persistent/chronic disease. Patient and physicians were largely aligned on diagnosis, symptoms, and treatment use. I-WISh demonstrated that patients and physicians largely align on overall ITP symptom burden, with certain differences, for example, fatigue. Understanding the emotional and clinical toll of ITP on the patient will facilitate shared decision-management, setting and establishment of treatment goals and disease stage-appropriate treatment selection.
© 2020 The Authors. American Journal of Hematology published by Wiley Periodicals LLC.
Conflict of interest statement
N. Cooper reports honoraria for speaking engagements and advisory boards from Amgen and Novartis. C. Kruse received honoraria for speaking engagements and consultancy fees paid to PDSA from Amgen, Novartis, and Rigel Pharmaceuticals. S. Watson reports advisory work for Novartis. M. Morgan, reports advisory work for Novartis, Sobi and UCB. D. Provan received research grants and honoraria from Novartis and Amgen and consultancy for UCB, MedImmune, and ONO Pharmaceutical. W. Ghanima received research grants from Bayer, BMS, and Novartis and honoraria for participation in advisory boards for Amgen and Novartis. D. M. Arnold received research grants from Novartis, Amgen, and Bristol‐Myers Squibb and worked as a consultant for Amgen, Novartis, Rigel, and Principia. Y. Tomiyama reports honoraria and membership of advisory committees for Novartis and honoraria from Chugai and Kyowa‐Kirin. C. Santoro reports participating in speakers' bureaus for Amgen, advisory boards for Grifols and Gilead, and speakers' bureaus and advisory boards for Shire/Takeda, Novo Nordisk, Bayer, Pfizer, CSL, Roche, Novartis and Sobi. M. Michel reports membership of advisory boards and speaker engagements for Amgen and Novartis. B. Lovrencic reports honoraria for consultancy fee paid to Aipit (Italian Association of Immune Thrombocytopenic Purpura) from UCB and Novartis. J.B. Bussel reports consulting or membership on an advisory board for Amgen, Novartis, Dova, Rigel, UCB, Argenx, Kezar, RallyBIo, and Momenta; honoraria from UptoDate, and participating in a speakers' bureau for Novartis, 3S (Shenyang) Bio, and Physician Education Resource. J. Haenig is a full‐time employee of Novartis Pharma AG. T. Bailey and G. Taylor‐Stokes are employees of Adelphi Real World, which has received consultancy fees from Novartis. A. Kruse, S. Laborde, and M. Hou have nothing to disclose.
Figures
Comment in
-
I-WISh: A wish list for immune thrombocytopenia quality of life indicators becomes reality.Am J Hematol. 2021 Feb 1;96(2):172-173. doi: 10.1002/ajh.26053. Epub 2020 Dec 10. Am J Hematol. 2021. PMID: 33219702 No abstract available.
References
-
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386‐2393. - PubMed
-
- Matzdorff A, Beer JH. Immune thrombocytopenia patients requiring anticoagulation–maneuvering between Scylla and Charybdis. Semin Hematol. 2013;50(Suppl 1):S83‐S88. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
