Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients
- PMID: 33171020
- PMCID: PMC7613119
- DOI: 10.1111/ajt.16395
Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients
Abstract
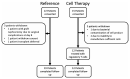
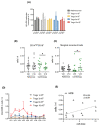
Short-term outcomes in kidney transplantation are marred by progressive transplant failure and mortality secondary to immunosuppression toxicity. Immune modulation with autologous polyclonal regulatory T cell (Treg) therapy may facilitate immunosuppression reduction promoting better long-term clinical outcomes. In a Phase I clinical trial, 12 kidney transplant recipients received 1-10 × 106 Treg per kg at Day +5 posttransplantation in lieu of induction immunosuppression (Treg Therapy cohort). Nineteen patients received standard immunosuppression (Reference cohort). Primary outcomes were rejection-free and patient survival. Patient and transplant survival was 100%; acute rejection-free survival was 100% in the Treg Therapy versus 78.9% in the reference cohort at 48 months posttransplant. Treg therapy revealed no excess safety concerns. Four patients in the Treg Therapy cohort had mycophenolate mofetil withdrawn successfully and remain on tacrolimus monotherapy. Treg infusion resulted in a long-lasting dose-dependent increase in peripheral blood Tregs together with an increase in marginal zone B cell numbers. We identified a pretransplantation immune phenotype suggesting a high risk of unsuccessful ex-vivo Treg expansion. Autologous Treg therapy is feasible, safe, and is potentially associated with a lower rejection rate than standard immunosuppression. Treg therapy may provide an exciting opportunity to minimize immunosuppression therapy and improve long-term outcomes.
Keywords: clinical research/practice; clinical trial; immune regulation; immunosuppression/immune modulation; immunosuppressive regimens - minimization/withdrawal; kidney transplantation/nephrology; kidney transplantation: living donor; monitoring: immune; translational research/science.
© 2020 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts of interest to disclose as described by the
Figures




References
-
- Rao NN, Coates PT. Cardiovascular disease after kidney transplant. Semin Nephrol. 2018;38(3):291–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

