Sample pooling on the Cepheid Xpert® Xpress SARS-CoV-2 assay
- PMID: 33171384
- PMCID: PMC7558241
- DOI: 10.1016/j.diagmicrobio.2020.115238
Sample pooling on the Cepheid Xpert® Xpress SARS-CoV-2 assay
Abstract
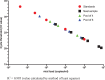
The COVID-19 pandemic has placed unprecedented global demand on laboratory supplies required for testing. Sample pooling has been investigated by laboratories as a strategy to preserve testing capacity. We evaluate the performance of Cepheid Xpert® Xpress SARS-CoV-2 RT-PCR assay for testing samples in pools of 4 and 6. Clinical samples containing SARS-CoV-2, and confirmed negative clinical samples were used to create sample pools. Clinical samples had 'neat' Xpert® E gene cycle threshold values ranging between 20 and 28 and all were detected qualitatively when contained in pools of 4 or 6 samples. For these samples, pooling had a median change in cycle threshold value of 2.0 in pools of 4, and of 2.9 in pools of 6. With the use of Cepheid Xpert® Xpress SARS-CoV-2 RT-PCR assay, pooling of 4 or 6 samples may be an effective strategy to increase testing capacity.
Keywords: Cepheid; GeneXpert; Pooling; SARS-CoV-2.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous