Sirtuin 6 deficiency induces endothelial cell senescence via downregulation of forkhead box M1 expression
- PMID: 33171439
- PMCID: PMC7695388
- DOI: 10.18632/aging.202176
Sirtuin 6 deficiency induces endothelial cell senescence via downregulation of forkhead box M1 expression
Abstract
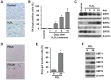
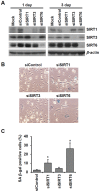
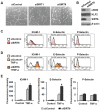
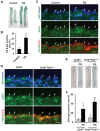
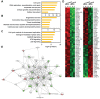
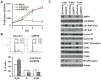
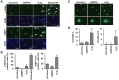
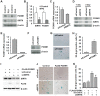
Cellular senescence of endothelial cells causes vascular dysfunction, promotes atherosclerosis, and contributes to the development of age-related vascular diseases. Sirtuin 6 (SIRT6), a conserved NAD+-dependent protein deacetylase, has beneficial effects against aging, despite the fact that its functional mechanisms are largely uncharacterized. Here, we show that SIRT6 protects endothelial cells from senescence. SIRT6 expression is progressively decreased during both oxidative stress-induced senescence and replicative senescence. SIRT6 deficiency leads to endothelial dysfunction, growth arrest, and premature senescence. Using genetically engineered endothelial cell-specific SIRT6 knockout mice, we also show that down-regulation of SIRT6 expression in endothelial cells exacerbates vascular aging. Expression microarray analysis demonstrated that SIRT6 modulates the expression of multiple genes involved in cell cycle regulation. Specifically, SIRT6 appears to regulate the expression of forkhead box M1 (FOXM1), a critical transcription factor for cell cycle progression and senescence. Overexpression of FOXM1 ameliorates SIRT6 deficiency-induced endothelial cell senescence. In this work, we demonstrate the role of SIRT6 as an anti-aging factor in the vasculature. These data may provide the basis for future novel therapeutic approaches against age-related vascular disorders.
Keywords: FOXM1; SIRT6; cell cycle; endothelial cell; senescence.
Conflict of interest statement
Figures
References
-
- van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, Smulders YM, Twisk JW, Stehouwer CD. Endothelial dysfunction and low-grade inflammation are associated with greater arterial stiffness over a 6-year period. Hypertension. 2011; 58:588–95. 10.1161/HYPERTENSIONAHA.111.174557 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

