A Cell-Based ELISA to Improve the Serological Analysis of Anti-SARS-CoV-2 IgG
- PMID: 33171590
- PMCID: PMC7695166
- DOI: 10.3390/v12111274
A Cell-Based ELISA to Improve the Serological Analysis of Anti-SARS-CoV-2 IgG
Abstract
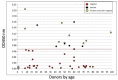
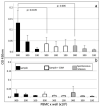
Knowledge of the antibody-mediated immune response to SARS-CoV-2 is crucial to understand virus immunogenicity, establish seroprevalence, and determine whether subjects or recovered patients are at risk for infection/reinfection and would therefore benefit from vaccination. Here, we describe a novel and simple cell-ELISA specifically designed to measure viral spike S1-specific IgG produced in vitro by B cells in peripheral blood mononuclear cell (PBMC) cultures from a cohort of 45 asymptomatic (n = 24) and symptomatic (n = 21) individuals, with age ranging from 8 to 99 years. All subjects underwent ELISA serological screening twice, at the same time as the cell-ELISA (T2) as well as 35-60 days earlier (T1). Cryopreserved PBMCs of healthy donors obtained years before the COVID-19 pandemic were also included in the analysis. The preliminary results presented here show that out of 45 tested subjects, 16 individuals (35.5%) were positive to the cell-ELISA, 11 (24.5%) were concomitantly positive in the serological screening (T1 and/or T2), and only one person was exclusively positive in ELISA (T1) and negative in cell-ELISA, though values were close to the cutoff. Of note, five individuals (11.2%) tested negative in ELISA but positive in cell-ELISA and thus, they appear to have circulating B cells that produce antibodies against SARS-CoV-2, likely at levels that are undetectable in the serum, which challenges the negative results of the serological screening. The relative level of in vitro secreted IgG was measurable in positive subjects, ranging from 7 to 50 ng/well. Accordingly, all anti-SARS-CoV-2 antibody-positive subjects previously reported moderate to severe symptoms attributable to COVID-19, even though the RT-PCR data were rarely available to confirm viral infection. Overall, the described cell-ELISA might be an effective method for detecting subjects who encountered the virus in the past, and thus helpful to improve serological ELISA tests in the case of undetectable/equivocal circulating IgG levels, and a suitable and improved tool to better evaluate SARS-CoV-2-specific humoral immunity in the COVID-19 pandemic.
Keywords: B cell memory; COVID-19; SARS-CoV-2; cell-ELISA; in vitro IgG; spike S1 protein.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures
References
-
- Halliley J.L., Kyu S., Kobie J.J., Walsh E.E., Falsey A.R., Randall T.D., Treanor J., Feng C., Sanz I., Lee F.E.-H. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–3587. doi: 10.1016/j.vaccine.2010.02.088. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

