Twenty Years of Equine Piroplasmosis Research: Global Distribution, Molecular Diagnosis, and Phylogeny
- PMID: 33171698
- PMCID: PMC7695325
- DOI: 10.3390/pathogens9110926
Twenty Years of Equine Piroplasmosis Research: Global Distribution, Molecular Diagnosis, and Phylogeny
Abstract
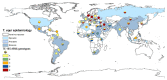
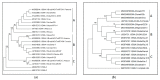
Equine piroplasmosis (EP), caused by the hemoparasites Theileria equi, Theileria haneyi, and Babesia caballi, is an important tick-borne disease of equines that is prevalent in most parts of the world. Infection may affect animal welfare and has economic impacts related to limitations in horse transport between endemic and non-endemic regions, reduced performance of sport horses and treatment costs. Here, we analyzed the epidemiological, serological, and molecular diagnostic data published in the last 20 years, and all DNA sequences submitted to GenBank database, to describe the current global prevalence of these parasites. We demonstrate that EP is endemic in most parts of the world, and that it is spreading into more temperate climates. We emphasize the importance of using DNA sequencing and genotyping to monitor the spread of parasites, and point to the necessity of further studies to improve genotypic characterization of newly recognized parasite species and strains, and their linkage to virulence.
Keywords: Babesia caballi; Theileria equi; equine; equine piroplasmosis; genotyping.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Friedhoff K.T., Tenter A.M., Muller I. Haemoparasites of equines: Impact on international trade of horses. Rev. Sci. Tech. 1990;9:1187–1194. - PubMed
-
- Rothschild C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013;23:115–120. doi: 10.1016/j.jevs.2013.03.189. - DOI
-
- Knowles D.P., Kappmeyer L.S., Haney D., Herndon D.R., Fry L.M., Munro J.B., Sears K., Ueti M.W., Wise L.N., Silva M., et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018;48:679–690. doi: 10.1016/j.ijpara.2018.03.010. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

