Temporal Persistence of Bromadiolone in Decomposing Bodies of Common Kestrel (Falco tinnunculus)
- PMID: 33171863
- PMCID: PMC7711720
- DOI: 10.3390/toxics8040098
Temporal Persistence of Bromadiolone in Decomposing Bodies of Common Kestrel (Falco tinnunculus)
Abstract
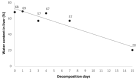
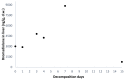
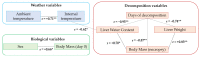
Bromadiolone is a second generation anticoagulant rodenticide (SGAR) used to control pest rodents worldwide. SGARs are frequently involved in secondary poisoning in rodent predators due to their persistence and toxicity. This study aims to evaluate the persistence of bromadiolone in liver at different stages of carcass decomposition in experimentally-dosed common kestrels (Falco tinnunculus) to understand the possibility of detecting bromadiolone in cases of wildlife poisoning and the potential risk of tertiary poisoning. Twelve individuals were divided into the bromadiolone-dose group (dosed with 55 mg/kg b.w) and the control group. Hepatic bromadiolone concentrations found in each stage of decomposition were: 3000, 2891, 4804, 4245, 8848, and 756 ng/g dry weight at 1-2 h (fresh carcass), 24 h (moderate decomposition), 72 h, 96 h (advanced decomposition), seven days (very advanced decomposition), and 15 days (initial skeletal reduction) after death, respectively. Liver bromadiolone concentrations in carcasses remained relatively stable over the first four days and raised on day 7 of decomposition under the specific conditions of this experiment, presenting a risk of causing tertiary poisoning. However, at the initial skeletal reduction stage, liver bromadiolone concentration declined, which should be considered to interpret toxicological analyses and for proper diagnosis. This experimental study provides for the first time some light to better understand the degradation of SGARs in carcasses in the wild.
Keywords: anticoagulant rodenticides; biomonitoring; bromadiolone degradation; carcass decomposition; wildlife poisoning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Berny P., Buronfosse T., Lorgue G. Anticoagulant Poisoning in Animals: A Simple New High-Performance Thin-Layer Chromatographic (HPTLC) Method for the Simultaneous Determination of Eight Anticoagulant Rodenticides in Liver Samples. J. Anal. Toxicol. 1995;19:576–580. doi: 10.1093/jat/19.7.576. - DOI - PubMed
-
- Hosea R.C. Exposure of non-target wildlife to anticoagulant rodenticides in California. Proc. Vertebr. Pest Conf. 2000;19 doi: 10.5070/v419110029. - DOI
-
- Ruiz-Suárez N., Henríquez-Hernández L.A., Valerón P.F., Boada L.D., Zumbado M., Camacho M., Almeida-González M., Luzardo O.P. Assessment of anticoagulant rodenticide exposure in six raptor species from the Canary Islands (Spain) Sci. Total Environ. 2014;485–486:371–376. doi: 10.1016/j.scitotenv.2014.03.094. - DOI - PubMed
-
- van den Brink N.W., Elliott J.E., Shore R.F., Rattner B.A. Anticoagulant Rodenticides and Wildlife: Concluding Remarks. Springer; Berlin/Heidelberg, Germany: 2018. pp. 379–386.
Grants and funding
LinkOut - more resources
Full Text Sources

