Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer
- PMID: 33171939
- PMCID: PMC7664196
- DOI: 10.3390/ijms21218323
Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer
Abstract
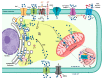
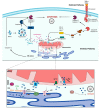
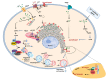
Calcium (Ca2+) is a major second messenger in cells and is essential for the fate and survival of all higher organisms. Different Ca2+ channels, pumps, or exchangers regulate variations in the duration and levels of intracellular Ca2+, which may be transient or sustained. These changes are then decoded by an elaborate toolkit of Ca2+-sensors, which translate Ca2+ signal to intracellular operational cell machinery, thereby regulating numerous Ca2+-dependent physiological processes. Alterations to Ca2+ homoeostasis and signaling are often deleterious and are associated with certain pathological states, including cancer. Altered Ca2+ transmission has been implicated in a variety of processes fundamental for the uncontrolled proliferation and invasiveness of tumor cells and other processes important for cancer progression, such as the development of resistance to cancer therapies. Here, we review what is known about Ca2+ signaling and how this fundamental second messenger regulates life and death decisions in the context of cancer, with particular attention directed to cell proliferation, apoptosis, and autophagy. We also explore the intersections of Ca2+ and the therapeutic targeting of cancer cells, summarizing the therapeutic opportunities for Ca2+ signal modulators to improve the effectiveness of current anticancer therapies.
Keywords: apoptosis; autophagy; calcium; cancer; cell cycle; chemotherapy; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115:599–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

