The Effects of Non-Nutritive Artificial Sweeteners, Aspartame and Sucralose, on the Gut Microbiome in Healthy Adults: Secondary Outcomes of a Randomized Double-Blinded Crossover Clinical Trial
- PMID: 33171964
- PMCID: PMC7694690
- DOI: 10.3390/nu12113408
The Effects of Non-Nutritive Artificial Sweeteners, Aspartame and Sucralose, on the Gut Microbiome in Healthy Adults: Secondary Outcomes of a Randomized Double-Blinded Crossover Clinical Trial
Abstract
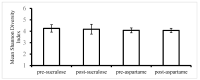
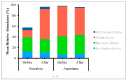
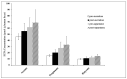
Non-nutritive artificial sweeteners (NNSs) may have the ability to change the gut microbiota, which could potentially alter glucose metabolism. This study aimed to determine the effect of sucralose and aspartame consumption on gut microbiota composition using realistic doses of NNSs. Seventeen healthy participants between the ages of 18 and 45 years who had a body mass index (BMI) of 20-25 were selected. They undertook two 14-day treatment periods separated by a four-week washout period. The sweeteners consumed by each participant consisted of a standardized dose of 14% (0.425 g) of the acceptable daily intake (ADI) for aspartame and 20% (0.136 g) of the ADI for sucralose. Faecal samples collected before and after treatments were analysed for microbiome and short-chain fatty acids (SCFAs). There were no differences in the median relative proportions of the most abundant bacterial taxa (family and genus) before and after treatments with both NNSs. The microbiota community structure also did not show any obvious differences. There were no differences in faecal SCFAs following the consumption of the NNSs. These findings suggest that daily repeated consumption of pure aspartame or sucralose in doses reflective of typical high consumption have minimal effect on gut microbiota composition or SCFA production.
Keywords: aspartame; gut microbiome; non-nutritive sweetener; protocol; randomized clinical trial; sucralose.
Conflict of interest statement
D.M. was an invited speaker at a seminar entitled ‘Conflicting Outcomes from Systematic Reviews: Is the Consumption of Low-Calorie Sweeteners a Benefit or a Risk for Weight Management?’ at Nutrition 2018 in Boston, Massachusetts, USA, which was sponsored by PepsiCo. PepsiCo paid for his accommodation, conference fee and honorarium. PepsiCo is a company that sells products that contain non-nutritive sweeteners. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Gardner C., Wylie-Rosett J., Gidding S.S., Steffen L.M., Johnson R.K., Reader D., Lichtenstein A.H. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2012;126:509–519. doi: 10.1161/CIR.0b013e31825c42ee. - DOI - PubMed
-
- Government of Canada List of Permitted Sweeteners (Lists of Permitted Food Additives) [(accessed on 3 April 2019)]; Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safe....
-
- Garriguet D. Beverage consumption of Canadian adults. Health Rep. 2008;19:23–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

