Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing
- PMID: 33172010
- PMCID: PMC7664222
- DOI: 10.3390/ma13215005
Physicochemical Properties and Cell Viability of Shrimp Chitosan Films as Affected by Film Casting Solvents. I-Potential Use as Wound Dressing
Abstract
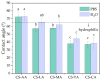
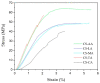
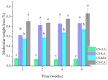
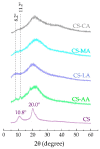
Chitosan solubility in aqueous organic acids has been widely investigated. However, most of the previous works have been done with plasticized chitosan films and using acetic acid as the film casting solvent. In addition, the properties of these films varied among studies, since they are influenced by different factors such as the chitin source used to produce chitosan, the processing variables involved in the conversion of chitin into chitosan, chitosan properties, types of acids used to dissolve chitosan, types and amounts of plasticizers and the film preparation method. Therefore, this work aimed to prepare chitosan films by the solvent casting method, using chitosan derived from Litopenaeus vannamei shrimp shell waste, and five different organic acids (acetic, lactic, maleic, tartaric, and citric acids) without plasticizer, in order to evaluate the effect of organic acid type and chitosan source on physicochemical properties, degradation and cytotoxicity of these chitosan films. The goal was to select the best suited casting solvent to develop wound dressing from shrimp chitosan films. Shrimp chitosan films were analyzed in terms of their qualitative assessment, thickness, water vapor permeability (WVP), water vapor transmission rate (WVTR), wettability, tensile properties, degradation in phosphate buffered saline (PBS) and cytotoxicity towards human fibroblasts using the resazurin reduction method. Regardless of the acid type employed in film preparation, all films were transparent and slightly yellowish, presented homogeneous surfaces, and the thickness was compatible with the epidermis thickness. However, only the ones prepared with maleic acid presented adequate characteristics of WVP, WVTR, wettability, degradability, cytotoxicity and good tensile properties for future application as a wound dressing material. The findings of this study contributed not only to select the best suited casting solvent to develop chitosan films for wound dressing but also to normalize a solubilization protocol for chitosan, derived from Litopenaeus vannamei shrimp shell waste, which can be used in the pharmaceutical industry.
Keywords: chitosan; film; organic acids; solubility; solvent casting; wound dressing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effect of the molar mass of chitosan and film casting solvents on the properties of chitosan films loaded with Mentha spicata essential oil for potential application as wound dressing.J Biomater Sci Polym Ed. 2024 Dec;35(18):2807-2828. doi: 10.1080/09205063.2024.2390752. Epub 2024 Aug 21. J Biomater Sci Polym Ed. 2024. PMID: 39167543
-
Evaluation of the Mechanical Properties and Drug Permeability of Chitosan/Eudragit RL Composite Film.Osong Public Health Res Perspect. 2015 Feb;6(1):14-9. doi: 10.1016/j.phrp.2014.12.001. Epub 2014 Dec 18. Osong Public Health Res Perspect. 2015. PMID: 25737826 Free PMC article.
-
Effect of molecular weight, acid, and plasticizer on the physicochemical and antibacterial properties of β-chitosan based films.J Food Sci. 2012 May;77(5):E127-36. doi: 10.1111/j.1750-3841.2012.02686.x. J Food Sci. 2012. PMID: 23163939
-
The Effects of Curing and Casting Methods on the Physicochemical Properties of Polymer Films.AAPS PharmSciTech. 2018 Aug;19(6):2740-2749. doi: 10.1208/s12249-018-1113-1. Epub 2018 Jul 5. AAPS PharmSciTech. 2018. PMID: 29978291
-
Chitosan films for regenerative medicine: fabrication methods and mechanical characterization of nanostructured chitosan films.Biophys Rev. 2019 Oct;11(5):807-815. doi: 10.1007/s12551-019-00591-6. Epub 2019 Sep 16. Biophys Rev. 2019. PMID: 31529358 Free PMC article. Review.
Cited by
-
Plasma-Initiated Grafting of Bioactive Peptide onto Nano-CuO/Tencel Membrane.Polymers (Basel). 2022 Oct 24;14(21):4497. doi: 10.3390/polym14214497. Polymers (Basel). 2022. PMID: 36365490 Free PMC article.
-
Current and Expected Trends for the Marine Chitin/Chitosan and Collagen Value Chains.Mar Drugs. 2023 Nov 23;21(12):605. doi: 10.3390/md21120605. Mar Drugs. 2023. PMID: 38132926 Free PMC article. Review.
-
Use of Piranha Solution as An Alternative Route to Promote Bioactivation of PEEK Surface with Low Functionalization Times.Molecules. 2022 Dec 22;28(1):74. doi: 10.3390/molecules28010074. Molecules. 2022. PMID: 36615270 Free PMC article.
-
Development of Bacterial Cellulose Biocomposites Combined with Starch and Collagen and Evaluation of Their Properties.Materials (Basel). 2021 Jan 19;14(2):458. doi: 10.3390/ma14020458. Materials (Basel). 2021. PMID: 33477891 Free PMC article.
-
Films for Wound Healing Fabricated Using a Solvent Casting Technique.Pharmaceutics. 2023 Jul 9;15(7):1914. doi: 10.3390/pharmaceutics15071914. Pharmaceutics. 2023. PMID: 37514100 Free PMC article. Review.
References
-
- Viseras C., Cerezo P., Sanchez R., Salcedo I., Aguzzi C. Current challenges in clay minerals for drug delivery. App. Clay. Sci. 2010;48:291–295. doi: 10.1016/j.clay.2010.01.007. - DOI
-
- Madhumathi K., Shalumon K., Rani V.D., Tamura H., Furuike T., Selvamurugan N., Nair S., Jayakumar R. Wet chemical synthesis of chitosan hydrogel–hydroxyapatite composite membranes for tissue engineering applications. Int. J. Biol. Macromol. 2009;45:12–15. doi: 10.1016/j.ijbiomac.2009.03.011. - DOI - PubMed
LinkOut - more resources
Full Text Sources

